365572
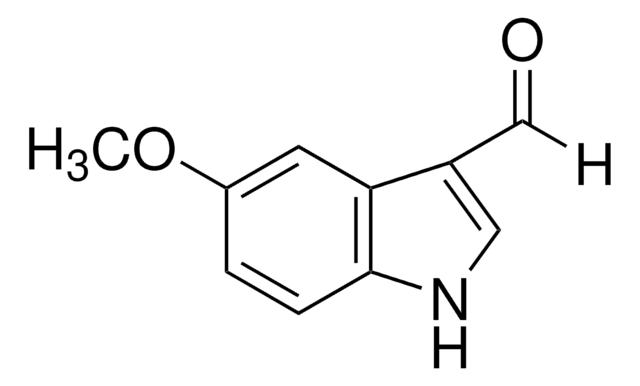
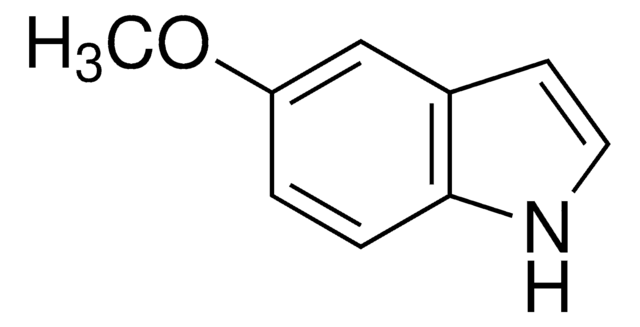
Methyl 6-methoxy-2-indolecarboxylate
99%
Sinónimos:
2-Methoxycarbonyl-6-methoxyindole, 6-Methoxyindole-2-carboxylic acid methyl ester
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H11NO3
Número de CAS:
Peso molecular:
205.21
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
99%
mp
117-119 °C (lit.)
grupo funcional
ester
cadena SMILES
COC(=O)c1cc2ccc(OC)cc2[nH]1
InChI
1S/C11H11NO3/c1-14-8-4-3-7-5-10(11(13)15-2)12-9(7)6-8/h3-6,12H,1-2H3
Clave InChI
OPUUCOLVBDQWEY-UHFFFAOYSA-N
Aplicación
Methyl 6-methoxy-2-indolecarboxylate is suitable for use in the production of dyes by Escherichia coli expressing naphthalene dioxygenase (NDO) and toluene dioxygenase (TDO). It is also suitable for use in the production of dyes by Escherichia coli expressing multicomponent phenol hydroxylase (mPH) from Pseudomonas sp. strains KL33 and KL28.
Reactant for preparation of:
- Benzoxazole containing indole analogs as peroxisome proliferator-activated receptor-γ/δ dual agonists
- Potent antiproliferative agent against human leukemia K562 cells
- Indole-indolone scaffold via [3+2] annulation of arynes
- Latonduine derivatives via intramolecular Heck reaction as possible anticancer agents
- Arylthioindoles as potent inhibitors of tubulin polymerization
- Heterocycle-fused derivatives of 1-oxo-1,2,3,4-tetrahydropyrazine via Ugi condensation
- Indole fatty alcohols (IFAs) as promoters of differentiation of neural stem cell derived neurospheres into neurons. Potential application for treatment of neurodegenerative diseases
- Light-dependent tumor necrosis factor-α antagonists
- 2-substituted indole melatonin receptor ligands
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
J Y Kim et al.
Letters in applied microbiology, 36(6), 343-348 (2003-05-20)
To isolate and characterize the phorate [O,O-diethyl-S-(ethylthio)methyl phosphoradiothioate] degrading bacteria from agricultural soil, and their assessment for multifarious biological activities of environmental and agronomic significance. Based on their morphological and biochemical characteristics, the selected isolates PS-1, PS-2 and PS-3 were
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico