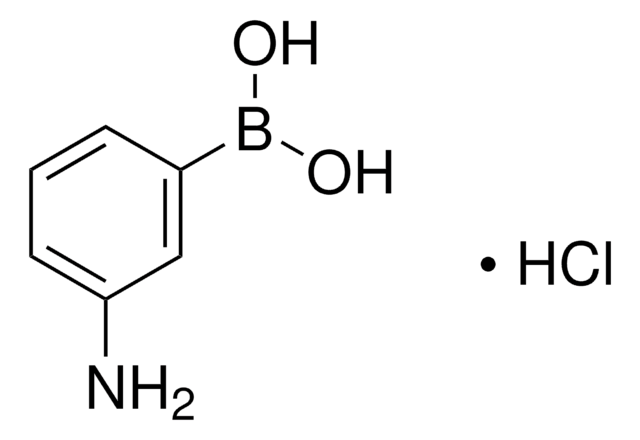
287512
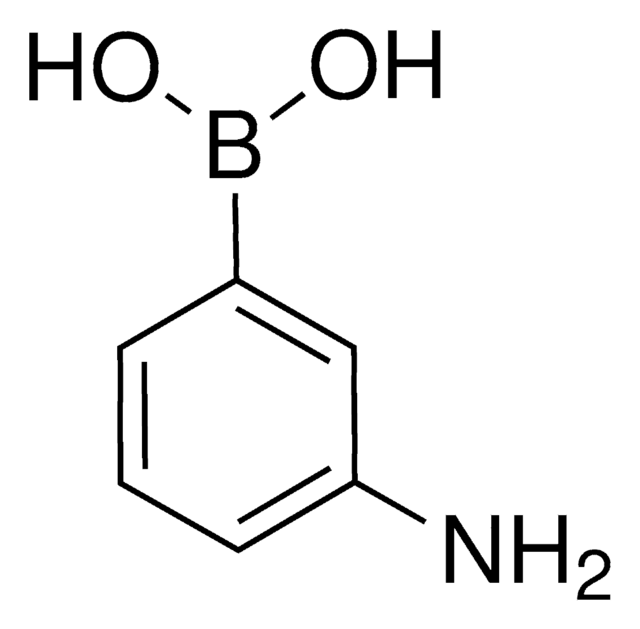
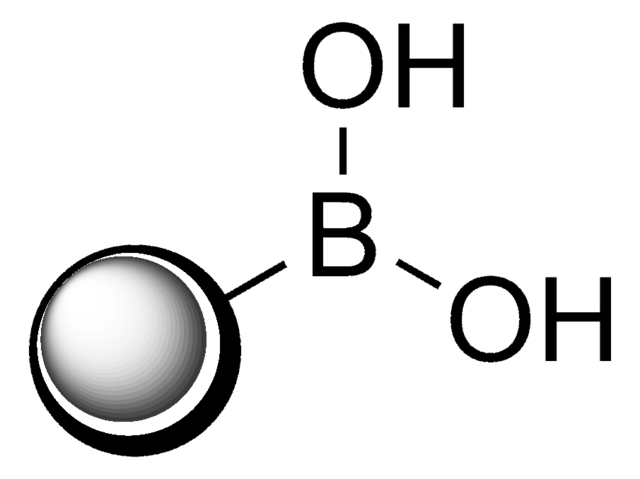
3-Aminophenylboronic acid monohydrate
98%
Sinónimos:
3-Aminobenzeneboronic acid
About This Item
Productos recomendados
Análisis
98%
formulario
solid
mp
93-96 °C (lit.)
cadena SMILES
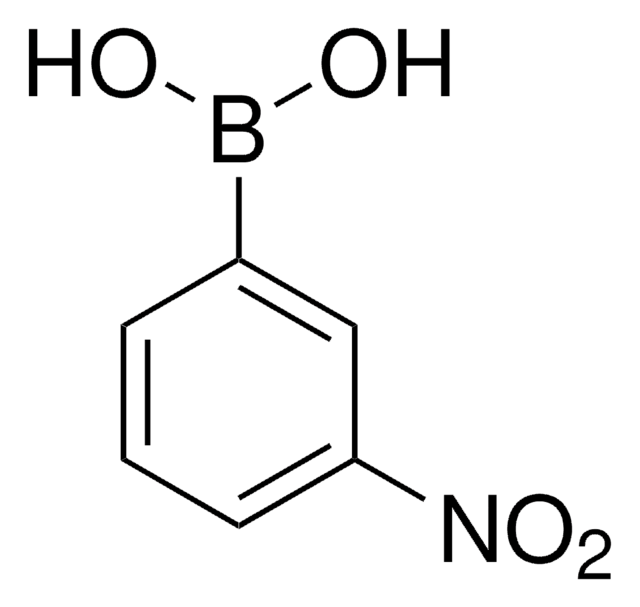
[H]O[H].Nc1cccc(c1)B(O)O
InChI
1S/C6H8BNO2.H2O/c8-6-3-1-2-5(4-6)7(9)10;/h1-4,9-10H,8H2;1H2
Clave InChI
XAEOVQODHLLNKX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Suzuki-Miyaura cross-coupling
Reagent used for Preparation of
- Gram-positive antivirulence drugs and inhibitors of Streptococcus agalactiae Stk1
- Regioisomer of Zaleplon (a sedative)
- Amphiphilic random glycopolymer, which self-assemble to form nanoparticles, with potential as a glucose-sensitive matrix
- Chemomechanical polymer that expands and contracts in blood plasma with high glucose selectivity
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico