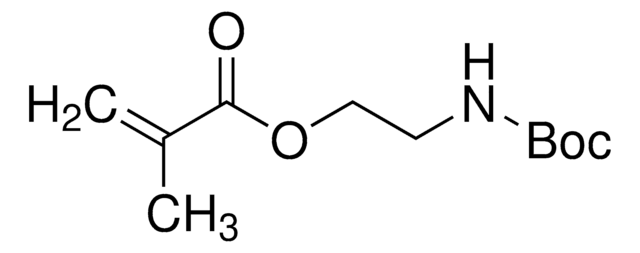
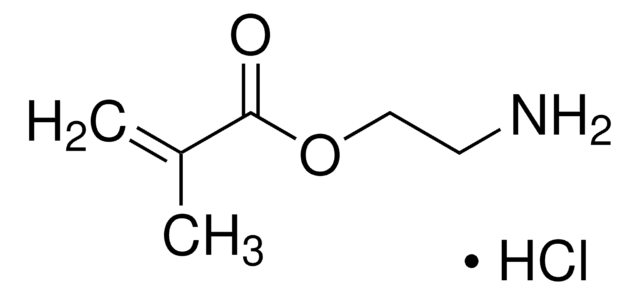
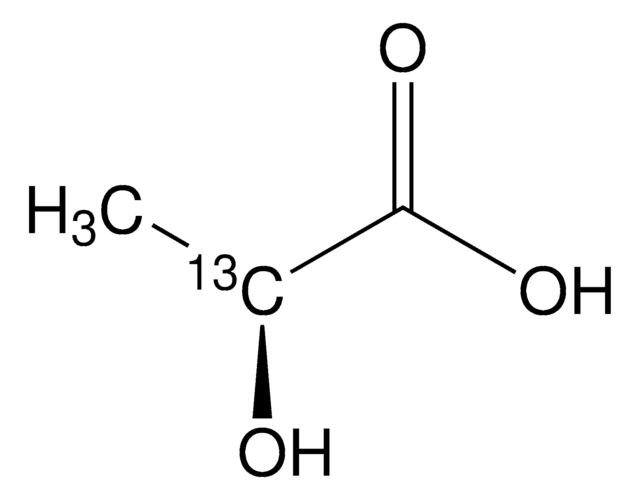
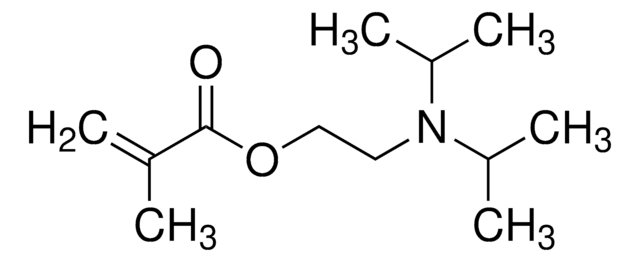
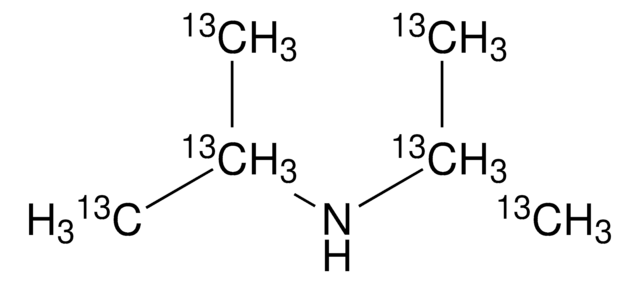
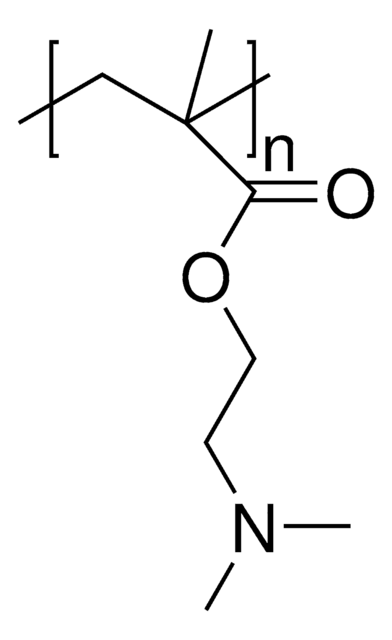
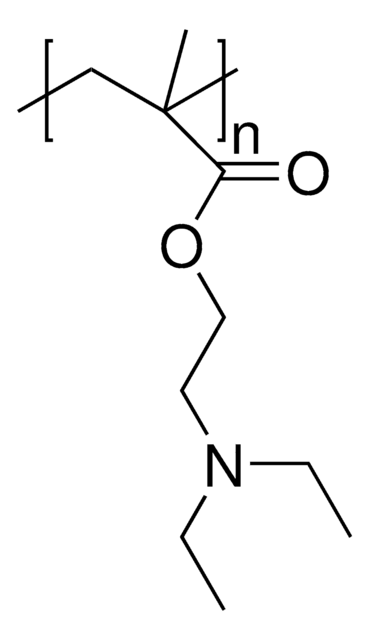
157872
1,3-Dithiane
97%
Sinónimos:
m-Dithiane (7CI), m-Dithiane (8CI)
Seleccione un Tamaño
563,00 PLN
Seleccione un Tamaño
About This Item
563,00 PLN
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
mp
52-54 °C (lit.)
grupo funcional
thioether
cadena SMILES
C1CSCSC1
InChI
1S/C4H8S2/c1-2-5-4-6-3-1/h1-4H2
Clave InChI
WQADWIOXOXRPLN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
1 of 4
Este artículo | 741612 | 909866 | 910104 |
|---|---|---|---|
| assay 97% | assay 97% (CP) | assay - | assay - |
| density 0.900 g/mL at 25 °C | density 0.764 g/mL at 25 °C | density - | density - |
| storage temp. 2-8°C | storage temp. - | storage temp. 2-8°C | storage temp. 2-8°C |
| form liquid | form liquid | form powder or solid | form powder or solid |
| refractive index n20/D 1.145 | refractive index - | refractive index - | refractive index - |
| contains ~100 ppm monomethyl ether hydroquinone as inhibitor | contains - | contains - | contains - |
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
194.0 °F - closed cup
Punto de inflamabilidad (°C)
90 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
This page shows how to perform a separation with a Sephadex column from GE Healthcare which are Size Exclusion Chromatography media consisting of an epichlorohydrin cross-linked dextran.
Ta strona pokazuje, jak przeprowadzić separację za pomocą kolumny Sephadex firmy Cytiva, która jest nośnikiem do chromatografii wykluczania składającym się z dekstranu usieciowanego epichlorohydryną.
Niniejszy protokół zawiera szczegółowe informacje na temat dokładnego pakowania różnych żywic chromatograficznych w puste kolumny do chromatografii wykluczania wielkości. Dołączona jest metoda oceny wydajności kolumny.
Column Packing and Preparation for Size Exclusion Chromatography
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![N-[3-(Dimethylamino)propyl]methacrylamide 99%, contains MEHQ as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/295/145/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951/640/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951.png)