5.74826
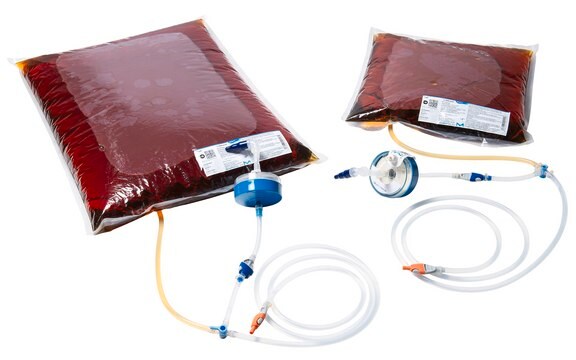
ReadyStream® Filter Set
For sterile filtration of water for convenient preparation of a large amounts of food testing media in a short time with the ReadyStream® workflow
Synonym(s):
0.22 µm filter, Filter Set, Sterile membrane filter
About This Item
Recommended Products
material
polyethersulfone filter
styrene-acrylonitrile (SAN) housing
sterility
sterile; γ-irradiated
feature
hydrophilic 2.7 mL
packaging
pack of 5 filter
technique(s)
sterile filtration: suitable
pore size
0.22 μm pore size
application(s)
food and beverages
compatibility
for use with ReadyStream® System
General description
Application
Other Notes
Legal Information
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
See how two water sources compare when preparing and performance testing microbiological culture media acc. to EN ISO 11133: centrally purified water vs. water from a Milli-Q® IX system were assessed. Tests included media specific for Listeria and Salmonella pathogens.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service