C-045
Cannabidiol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
CBD
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
SNAP-N-SPIKE®, SNAP-N-SHOOT®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
cannabis testing
cannabis testing
format
single component solution
storage temp.
−20°C
SMILES string
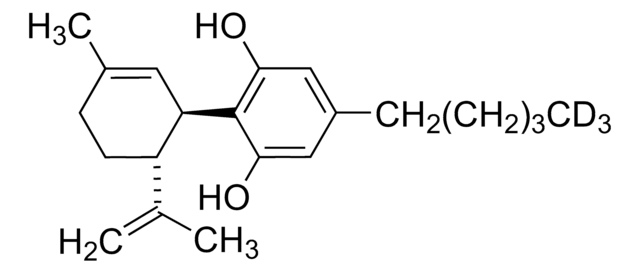
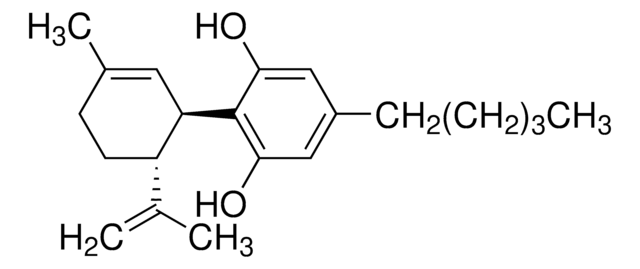
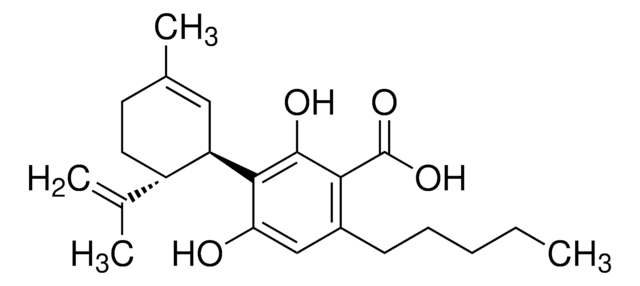
CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1
InChI
1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1
InChI key
QHMBSVQNZZTUGM-ZWKOTPCHSA-N
Gene Information
human ... CNR1(1268)
Looking for similar products? Visit Product Comparison Guide
General description
Cannabidiol (CBD) is one of the important cannabinoids found in plants of the Cannabaceae family known for its non-psychoactive properties. It forms upon the decarboxylation of its precursor cannabidiolic acid in the presence of light or heat. It shows various therapeutic properties, such as antioxidative, anti-inflammatory, antimicrobial, neuroprotective, anxiolytic, and anticonvulsant.
Application
- Determination of four cannabinoids from an oil matrix using reversed-phase high-performance liquid chromatography (RP-HPLC) combined with a diode array detector (DAD)
- Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) based technique to measure cannabidiol, ∆9-tetrahydrocannabinol, and its metabolites in whole blood samples obtained from rats administered a high dose of cannabidiol
- Simultaneous determination of cannabidiol and tetrahydrocannabinol in different hemp oil-containing products using isocratic RP-HPLC coupled with UV detection
- Multi-analysis of hair samples for Δ9-tetrahydrocannabinol, cannabinol, and cannabidiol by liquid-liquid extraction (LLE) followed by gas chromatography-mass spectrometry based quantification
- Ultrasound-assisted extraction of cannabidiol from three different commercial topical products followed by GC-MS based determination
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
High-performance liquid chromatography (HPLC) separation and quantitation of vitamin E acetate in commercially available vape products, in addition to simultaneous analysis of THC and CBD in the same run.
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabichromene solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
We offer a portfolio of cannabinoid CRMs such as THC and THCA, available as single component solutions or mixes, certified in accordance with ISO 17034 and ISO/IEC 17025 for accurate cannabinoid profiling and potency testing.
A comprehensive high performance liquid chromatography-diode array detection (HPLC-DAD) workflow for the analysis of 14 cannabinoids in hemp bud extracts within 10 minutes using robust Chromolith® monolithic silica HPLC columns with low column back pressure.
Protocols
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabidiolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromene solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabigerolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Δ9-Tetrahydrocannabinolic acid A (THCA-A)
THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabidiolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromene solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabigerolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Δ9-Tetrahydrocannabinolic acid A (THCA-A)
Rapid potency testing of marijuana-infused edibles using LC/MS on a biphenyl stationary phase detected eleven cannabinoids.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service