All Photos(4)
About This Item
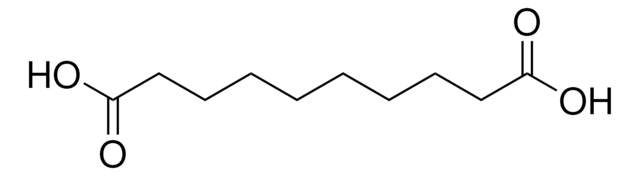
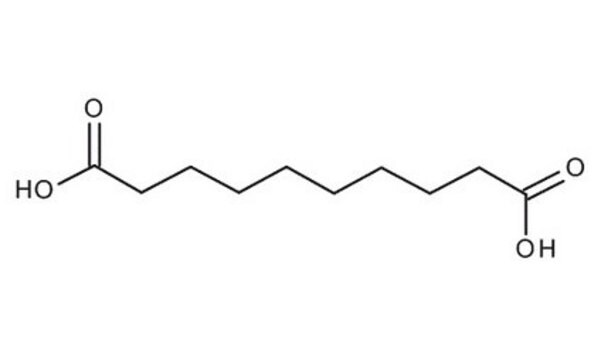
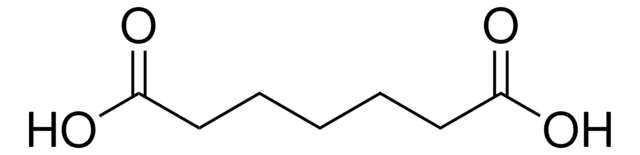
Linear Formula:
HOOC(CH2)6COOH
CAS Number:
Molecular Weight:
174.19
Beilstein:
1210161
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
230 °C/15 mmHg (lit.)
mp
139-146 °C
140-144 °C (lit.)
SMILES string
OC(=O)CCCCCCC(O)=O
InChI
1S/C8H14O4/c9-7(10)5-3-1-2-4-6-8(11)12/h1-6H2,(H,9,10)(H,11,12)
InChI key
TYFQFVWCELRYAO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Suberic acid can be used to synthesize:
- Perfluorinated analogs of suberoyl anilide hydroxamic acid (SAHA) as potent histone deacetylase inhibitors.
- Hemiester analogs of pregnenolone sulfate (PES).
- Cellulose acetate suberate, a cellulose ω-carboxyalkanoate.
- It can also act as a cross-linker for poly(vinyl alcohol) (PVA). The cross-linked molecule shows enhanced tensile strength when compared to neat PVA.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
410.0 °F - closed cup
Flash Point(C)
210 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Perfluorinated HDAC inhibitors as selective anticancer agents.
Walton J W, et al.
Organic & Biomolecular Chemistry, 15(43), 9186-9190 (2017)
Rifampin stability and solution concentration enhancement through amorphous solid dispersion in cellulose ω-carboxyalkanoate matrices.
Arca H C, et al.
Journal of Pharmaceutical Sciences, 107(1), 127-138 (2018)
Positive Modulators of the N-Methyl-d-aspartate Receptor: Structure?Activity Relationship Study of Steroidal 3-Hemiesters.
Krausova B, et al.
Journal of Medicinal Chemistry, 61(10), 4505-4516 (2018)
Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof.
Sonker A K, et al.
Journal of Polymers and the Environment, 26(5), 1782-1794 (2018)
Ronald J Nachman et al.
Peptides, 26(1), 115-120 (2005-01-01)
The aliphatic amino diacid alpha-aminosuberic acid can function as an effective, stable mimic of the hydrolysis-susceptible Tyr(SO3H) group in sulfakinin neuropeptide analogs for both hindgut contractile activity in cockroach and food intake-inhibition activity in the desert locust. In the analog
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service