HPLC Analysis of α-Casein Tryptic Digest on BIOshell A160 Peptide C18
Materials
standard
Casein from bovine milk
suitable for substrate for protein kinase (after dephosphorylation), purified powderCONDITIONS
sample/matrix
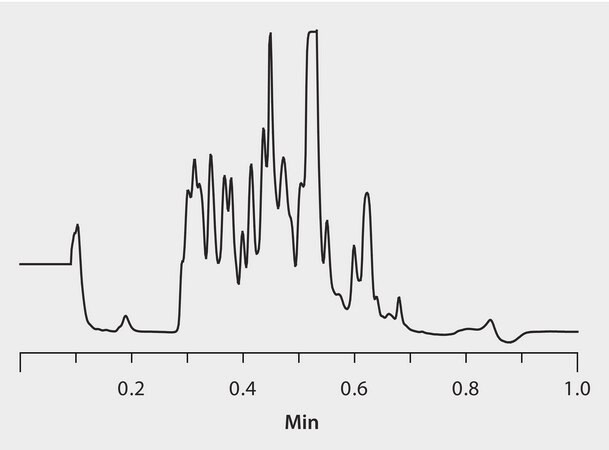
tryptic digested α-casein
column
BIOshell A160 Peptide C18, 5 cm x 4.6 mm I.D., 2.7 μm particles (66913-U)
mobile phase
[A] 0.1% TFA in water; [B] 0.1% TFA in water:acetonitilre (10:90, A:B)
gradient
0-0.05 min, 0-20% B; 0.05-0.40 min, to 40% B; 0.40-0.50 min, to 50% B; 0.50-0.69 min, to 90% B; 0.69-0.70 min, to 0% B; held for 0.3 min
flow rate
4 mL/min
column temp.
35 °C
detector
UV, 215 nm
injection
2 μL
Description
Analysis Note
LC-MS-based shotgun proteomics relies both on the power of the separation techniques, and the sensitivity of detection methods. As a viable alternative to classical approaches in this field, we developed a fully automated, comprehensive 2D LC system, in which RP LC x RP LC was coupled to MS detection, for the first time, and applied for the analysis of tryptic digests obtained from a-casein and dephosphorylated a–casein. The use of a significantly different pH in the two dimensions allowed to attain high peak capacity, despite the employment of novel identical stationary phases. Furthermore, such a combination addresses compatibility issues, thus allowing straightforward interfacing in on-line 2D LC configuration, as well as direct linkage to mass spectrometer. A theoretical peak capacity of approximately 8500 was calculated for the set-up employing four serially coupled C18 columns in the first dimension (4 columns, each 15 cm x 2.1 mm I.D., 2.7 μm particles), operated under basic conditions, and 3 cm length of the same stationary phase (3 cm x 4.6 mm I.D., 2.7 μm particles), under acidic conditions for fast second dimension analysis.
Other Notes
Sample Prep: one-tenth gram of α-casein was dissolved in 10 mL of 0.01 M ammonium formate buffer, and the pH was adjusted to 8.0 with ammonium hydroxide; the solution was heated in a boiling water bath for 6 min. After the solution cooled, 2.0 mg of trypsin from bovine pancreas was added, and the mixture was allowed to react for 4 h at 37 °C; the reaction was quenched by adding 0.1% trifluoroacetic acid to pH 2. The digest were stored at 4°C and filtered prior to injection through a 0.45 μm nylon membrane.
Legal Information
null