Palladium-catalyzed Cross-coupling Reactions
Troy Ryba, Ph.D., Product Manager
A variety of widely used palladium-catalyzed cross-coupling reactions can now be run under mild room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands. These transformations, including Suzuki-Miyaura, Sonogashira, Buchwald-Hartwig aminations, and Heck, are amongst the most heavily used bond forming reactions, both industrially and academically (Scheme 1).
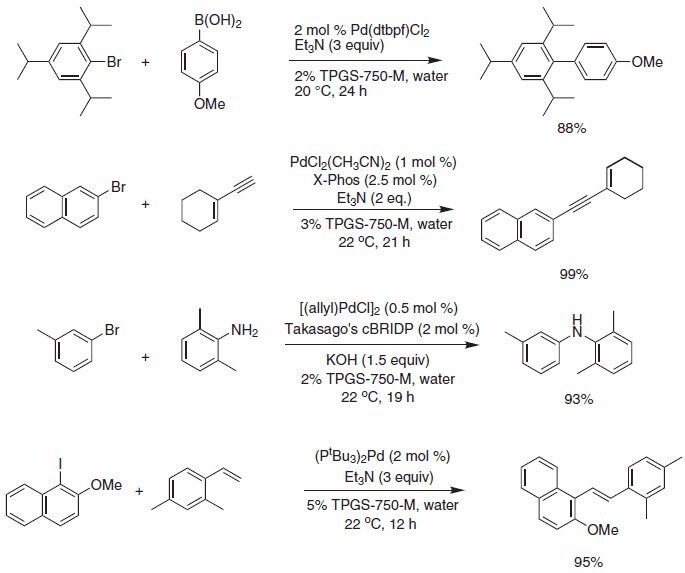
Scheme 1.Selected Pd-catalyzed cross coupling reactions.
Operationally extremely simple Suzuki-Miyaura reactions using micellar catalysis and bis(di-tert-butylphosphino)ferrocene palladium chloride complex provide access to highly sterically congested substrates at room temperature using triethylamine as base.
Sonogashira reactions and Buchwald-Hartwig aminations are also amenable to reaction in water with TPGS-750-M using the palladium chloride/X-Phos combination in the former, and allyl palladium chloride/ cBRIDP in the latter (Figure 1).
Heck cross-couplings with aryl iodides can be successfully performed using Pd(P(t-Bu)3)2 as the palladium source in the bulk aqueous environment containing TPGS-750-M (5 wt. %), obviating the need for high temperatures commonly associated with Heck reactions.
Zinc-mediated Negishi-like couplings between aryl and alkyl halides can be performed in aqueous TPGS-750-M (Scheme 2). Under these conditions, typically highly moisture sensitive organozinc halides are formed in situ from an alkyl halide and zinc dust, and react with an aryl halide under palladium catalysis. With the aid of a surfactant and a stabilizing ligand for RZnX, such as tetramethylethylenediamine (TMEDA), this entire process takes place in water, leading to a variety of primary and secondary alkyl-substituted aromatics. The choice of catalyst is crucial for the success of the reaction; Pd(Amphos)2Cl2 (Bis(di-tert-butyl (4-dimethylaminophenyl)phosphine) palladium(II) chloride) was found to be the optimal catalyst.

Scheme 2:Selected Negishi-like cross-coupling example.
More information on TPGS–750–M
To continue reading please sign in or create an account.
Don't Have An Account?