HPLC Analysis of Dermorphin in Horse Plasma on Ascentis® Express F5 after SPE with HybridSPE®
Materials
analytical column
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
horse plasma spiked at 10 μg/mL
SPE tube/cartridge
[TM"HybridSPE"]-Phospholipid Plate, 50mg/96-Well (575656-U)
sample addition
3:1, (1% formic acid in acetonitrile); plasma
column
Ascentis Express F5, 10 cm x 2.1 mm I.D., 2.7 μm particles (53569-U)
mobile phase
[A] acetonitrile [B] 20 mM ammonium formate; (90:10, A:B); pH 4.5 with formic acid, after mixing A & B
flow rate
0.4 mL/min
pressure
1305 psi (90 bar)
column temp.
35 °C
detector
MS, ESI(+), TIC, m/z 100-1000
injection
1 μL
Description
Analysis Note
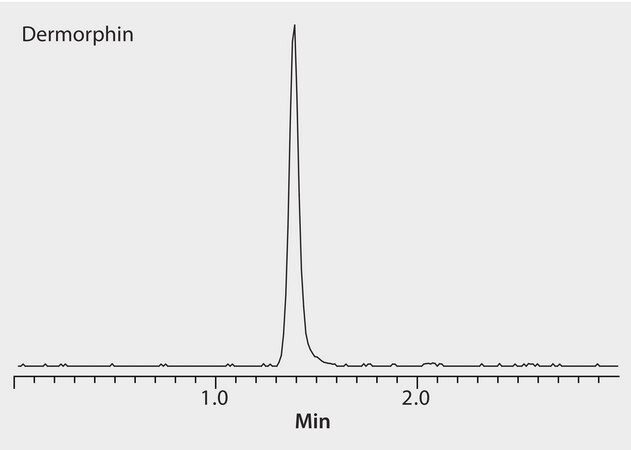
Dermorphin is a natural heptapeptide opioid. Because of poor recovery from horse plasma following protein precipitation, alternate methods of extraction are sought. While the HybridSPE protocol successfully removes proteins and phospholipids, dermorphin solubility in plasma remains a limitation. Dermorphin is effectively retained on the pentafluorophenyl phase under aqueous normal phase conditions.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany