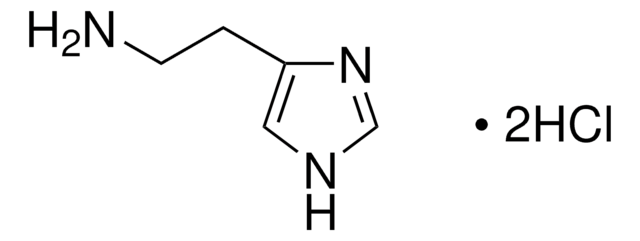
P6056
Endoproteinase Arg-C from mouse submaxillary gland
suitable for protein sequencing, lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.56
Recommended Products
form
lyophilized powder
Quality Level
packaging
vial of 5 μg
suitability
suitable for protein sequencing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Endoproteinase Arg-C from mouse submaxillary gland has been used to study the syncytium formation in MERS-CoV (middle East respiratory syndrome coronavirus)-infected Vero cells in the presence of exogenous proteases. It has been used for the digestion of Rpl23ab (ribosomal protein L23ab)-containing fraction for LC-MS (liquid chromatography–mass spectrometry)/MS analysis.
Biochem/physiol Actions
Endoproteinase Arg-C is a serine endoprotease from mouse submaxillary gland which hydrolyzes peptide bonds at the carboxyl side of arginyl residues. The enzyme has been shown to cleave Lys-Lys and Lys-Arg bonds, and all Arg-X bonds may not be hydrolyzed.
Unit Definition
One unit will hydrolyze 1.0 μmole of Nα-p-tosyl-L-arginine methyl ester per min at pH 8.0 at 25 °C.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2.
Shirato K, et al.
Journal of Virology, 87, 12552-12552 (2013)
Tanya R Porras-Yakushi et al.
The Journal of biological chemistry, 282(17), 12368-12376 (2007-03-01)
Ribosomal protein L23ab is specifically dimethylated at two distinct sites by the SET domain-containing enzyme Rkm1 in the yeast Saccharomyces cerevisiae. Using liquid column chromatography with electrospray-ionization mass spectrometry, we determined that Rpl23ab purified from the Deltarkm1 deletion strain demonstrated
Naoto Hoshi et al.
Molecular cell, 37(4), 541-550 (2010-03-02)
A-kinase anchoring proteins (AKAPs) coordinate cell signaling events. AKAP79 brings together different combinations of enzyme binding partners to customize the regulation of effector proteins. In neurons, muscarinic agonists mobilize an AKAP79-anchored pool of PKC that phosphorylates the KCNQ2 subunit of
Tanya R Porras-Yakushi et al.
The Journal of biological chemistry, 281(47), 35835-35845 (2006-09-29)
The ribosomal protein L12ab (Rpl12ab) in Saccharomyces cerevisiae is modified by methylation at both arginine and lysine residues. Although the enzyme responsible for the modification reaction at arginine 66 has been identified (Rmt2), the enzyme(s) responsible for the lysine modification(s)
Proteomics in Functional Genomics: Protein Structure Analysis (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service