I1406
Irinotecan hydrochloride
powder, ≥97% (HPLC)
Synonym(s):
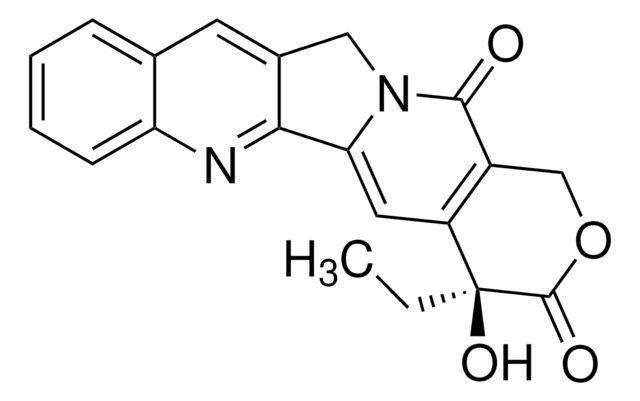
(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinolin-9-yl ester, CPT-11, [1,4′-Bipiperidine]-1′-carboxylic acid
About This Item
Recommended Products
product name
Irinotecan hydrochloride, topoisomerase inhibitor
biological source
plant (Fructus camptothecae)
Assay
≥97% (HPLC)
form
powder
solubility
DMSO: 50 mg/mL
storage temp.
2-8°C
SMILES string
Cl.CCc1c2CN3C(=O)C4=C(C=C3c2nc5ccc(OC(=O)N6CCC(CC6)N7CCCCC7)cc15)[C@@](O)(CC)C(=O)OC4
InChI
1S/C33H38N4O6.ClH/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2;/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3;1H/t33-;/m0./s1
InChI key
GURKHSYORGJETM-WAQYZQTGSA-N
Gene Information
human ... TOP1(7150)
Application
- in combination with 5-fluorouracil for screening growth inhibitory functionality in MDA-MB-231 breast cancer cells.
- in chemosensitivity screening of high-grade appendiceal (HGA) and low-grade appendiceal (LGA) organoids.
- as a chemotherapeutic agent in the cytotoxicity studies in combination with heat shock proteins inhibitors (HPSC1) in HT29 colon cancer cells.
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service