All Photos(1)
About This Item
Empirical Formula (Hill Notation):
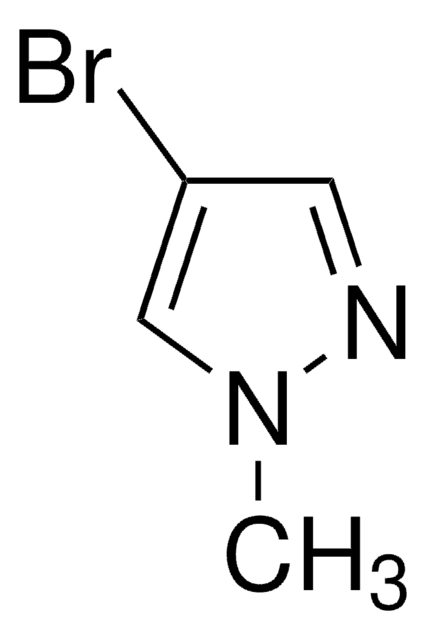
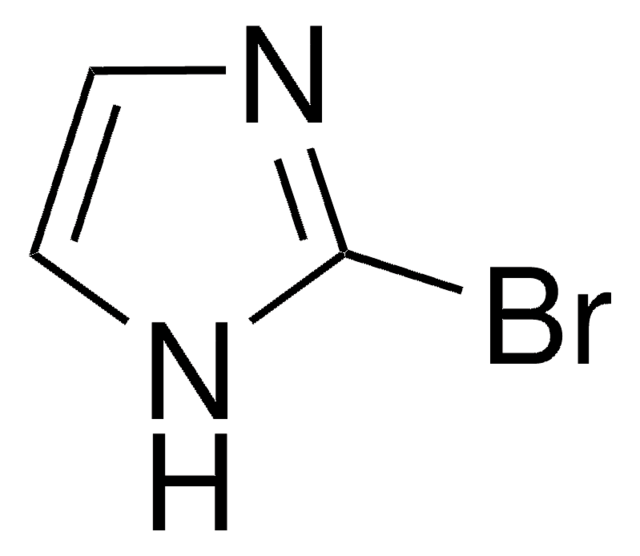
C3H3BrN2
CAS Number:
Molecular Weight:
146.97
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
250-260 °C (lit.)
mp
93-96 °C (lit.)
SMILES string
Brc1cn[nH]c1
InChI
1S/C3H3BrN2/c4-3-1-5-6-2-3/h1-2H,(H,5,6)
InChI key
WVGCPEDBFHEHEZ-UHFFFAOYSA-N
General description
4-Bromopyrazole is a heteroaryl halide and its cyanation in the presence of palladium catalysts has been reported.
4-Bromopyrazole is a pyrazole derivative. It is reported to react with titanium tetrachloride to afford binary adducts. Mutagenicity of 4-bromopyrazole has been tested using the L-arabinose forward mutation assay of Salmonella typhimurium. It is reported to inhibit the oxidative phosphorylation, the ATP-32P exchange reaction, and energy dependent and independent calcium uptake.
Application
4-Bromopyrazole may be used in the preparation of 4-bromo-1-(2-chloroethyl)-1H-pyrazole. It may be used as starting material in the synthesis of 1,4′-bipyrazoles.
4-Bromopyrazole may be used in the preparation of solid hexacoordinate complexes by reaction with dimethyl- and divinyl-tindichloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Alejandre-Durán et al.
Environmental mutagenesis, 8(4), 611-619 (1986-01-01)
The mutagenicity of pyrazole and seven pyrazole derivatives (4-nitropyrazole, 4-bromopyrazole, 1-methyl-4-nitropyrazole, 3,5-dimethyl-4-nitropyrazole, 1-methyl-4-bromopyrazole, 4,4'-dinitro-1, 1'-methylene-dipyrazole and 4,4'-dibromo-1,1'-methylene-dipyrazole) has been investigated with the L-arabinose forward mutation assay of Salmonella typhimurium. Two nitroimidazoles (1-methyl-5-nitroimidazole and metronidazole) were included as reference drugs. The
Inhibition of the oxidation of the urinary bladder carcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine by pyrazole and 4-substituted pyrazoles.
C C Irving et al.
Biochemical pharmacology, 37(8), 1642-1644 (1988-04-15)
Tetrahedron, 63, 748-748 (2007)
Dichlorodialkyltin complexes with 4-bromopyrazole. The crystal structure of bis(4-bromopyrazole- N2)dichlorodimethyltin(IV).
Casellato U, et al.
Journal of Organometallic Chemistry, 486(1-2), 105-107 (1995)
Ilia A. Guzei et al.
Inorganic chemistry, 36(20), 4415-4420 (2001-10-24)
Treatment of titanium tetrachloride with 3,5-di-tert-butylpyrazole affords the complexes [3,5-(C(CH(3))(3))(2)C(3)H(3)N(2)](2)[TiCl(6)] and (3,5-(C(CH(3))(3))(2)C(3)HN(2))(2)TiCl(2) in 37 and 42% yields, respectively. An analogous reaction with 3,5-dimethylpyrazole, 3-methylpyrazole, 4-bromopyrazole, and 4-iodopyrazole leads to the formation of corresponding TiCl(4)L(2) binary adducts in 30-86% yields. Crystal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service