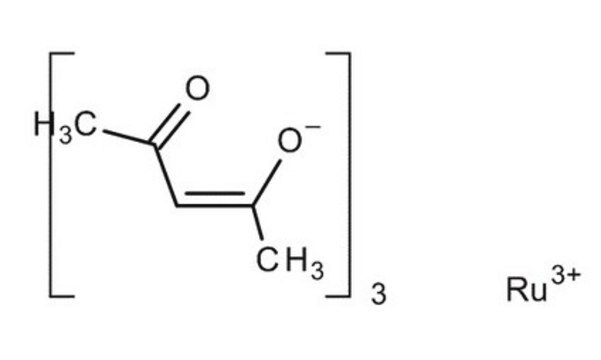
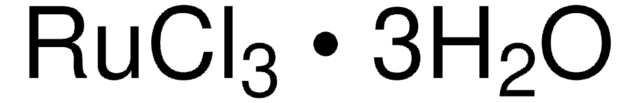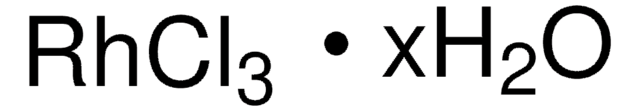208523
Ruthenium(III) chloride
Ru content 45-55%
Synonym(s):
Ruthenium trichloride
About This Item
Recommended Products
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Atom Transfer Radical Polymerization (ATRP)
density
3.11 g/mL at 25 °C (lit.)
SMILES string
Cl[Ru](Cl)Cl
InChI
1S/3ClH.Ru/h3*1H;/q;;;+3/p-3
InChI key
YBCAZPLXEGKKFM-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the synthesis of β‐amino alcohols by nucleophilic opening of epoxides with anilines.
- In the acetylation of varies of phenols, alcohols, thiols, and amines under mild conditions.
- In the synthesis of α‐aminonitriles by mixing aldehydes, amines, and trimethylsilyl cyanides.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
We presents an article about Copper(I)-mediated Living Radical Polymerization in the Presence of Pyridylmethanimine Ligands, and the emergence of living radical polymerization mediated by transition metal catalysts in 1995, which was a seminal piece of work in the field of synthetic polymer chemistry.
Applying ARGET ATRP to the Growth of Polymer Brush Thin Films by Surface-initiated Polymerization
Protocols
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
An article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service