An Introduction to Antibodies: Antibody-Antigen Interaction
Now that you know what an antigen and antibody are, let us consider the interaction between them. The strength of interaction between antibody and antigen at single antigenic sites can be described by the affinity of the antibody for the antigen. Within each antigenic site, the variable region of the antibody “arm” interacts through weak noncovalent forces with antigen at numerous sites. The greater the interaction, the stronger the affinity. Avidity is perhaps a more informative measure of the overall stability or strength of the antibody-antigen complex. It is controlled by three major factors: antibody epitope affinity, the valence of both the antigen and antibody, and the structural arrangement of the interacting parts. Ultimately these factors define the specificity of the antibody, that is, the likelihood that the particular antibody is binding to a precise antigen epitope.
Cross-reactivity refers to an antibody or population of antibodies binding to epitopes on other antigens. This can be caused either by low avidity or specificity of the antibody or by multiple distinct antigens having identical or very similar epitopes. Cross-reactivity is sometimes desirable when one wants general binding to a related group of antigens or when attempting cross-species labeling when the antigen epitope sequence is not highly conserved during evolution. Cross-reactivity can result in over- or under-estimation of the antigen concentration and is problematic in immunoassays. Immunochemical techniques capitalize upon the extreme specificity, at the molecular level, of each immunoglobulin for its antigen, even in the presence of high levels of contaminating molecules. The multivalency of most antigens and antibodies enables them to interact to form a precipitate. Examples of experimental applications that use antibodies are Western blot, immunohistochemistry and immunocytochemistry, enzyme-linked immunosorbent assay (ELISA), immunoprecipitation, and flow cytometry. Each is discussed in more detail in later sections of this reference guide.
Antibody-Antigen Interaction Kinetics
Nature of Antigen-Antibody Bonds
Factors Affecting Antigen-Antibody Reactions
Antibody-Antigen Interaction Kinetics
The specific association of antigens and antibodies is dependent on hydrogen bonds, hydrophobic interactions, electrostatic forces, and Van der Waals forces. These are of a weak, noncovalent nature, yet some of the associations between antigen and antibody can be quite strong. Like antibodies, antigens can be multivalent, either through multiple copies of the same epitope, or through the presence of multiple epitopes that are recognized by multiple antibodies. Interactions involving multivalency can produce more stabilized complexes; however, multivalency can also result in steric difficulties, thus reducing the possibility for binding. All antigen antibody binding is reversible and follows the basic thermodynamic principles of any reversible bimolecular interaction:
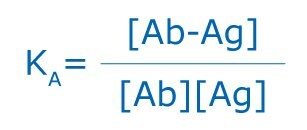
where KA is the affinity constant, [Ab-Ag] is the molar concentration of the antibody-antigen complex, and [Ab] and [Ag] are the molar concentrations of unoccupied binding sites on the antibody (Ab) or antigen (Ag), respectively.
The time taken to reach equilibrium is dependent on the rate of diffusion and the affinity of the antibody for the antigen and can vary widely. The affinity constant for antibody-antigen binding can span a wide range, extending from below 105/mol to above 1012/mol. Affinity constants can be affected by temperature, pH, and solvent. Affinity constants can be determined for monoclonal antibodies, but not for polyclonal antibodies, as multiple bond formations take place between polyclonal antibodies and their antigens. Quantitative measurements of antibody affinity for antigen can be made by equilibrium dialysis. Repeated equilibrium dialyses with a constant antibody concentration, but varying ligand concentration are used to generate Scatchard plots, which give information about affinity valence and possible cross-reactivity.
When designing experimental procedures, it is important to differentiate between monoclonal and polyclonal antibodies, as these differences are the foundation of both advantages and limitations of their use.
Nature of Antigen-Antibody Bonds
The combining site of an antibody is located in the F(ab) portion of the antibody molecule and is assembled from the hypervariable regions of the heavy and light chains. The binding between this site and the antigen takes place with the following characteristics and processes:
- The bonds that hold the antigen to the combining site of any antibody are noncovalent, and, hence, they are reversible in nature.
- These bonds may be hydrogen bonds, electrostatic bonds, or Van der Waals forces.
- Usually there are multiple bond formations observed, ensuring relatively tight binding between antibody and antigen.
- The specific binding between the antigenic determinant on the cell (known as epitope) and the antigen combining site (paratope) on the antibody involves very small portions of the molecules, usually comprising only a few amino acids.
- These sites are critical in antigen-antibody reactions as specific binding has to overcome repulsion between the two molecules.
- When the epitope comes in contact with paratope they are first attracted to each other by ionic and hydrophobic forces.
- These forces help them overcome their hydration energies and allow for the expulsion of water molecules as epitope and paratope approach each other.
- This attraction becomes even stronger when Van der Waals forces are employed later on to bring epitope and paratope even closer.
Factors Affecting Antigen-Antibody Reactions
The antigen-antibody reaction can be influenced by several factors. Some of the more common factors are:
Temperature
The optimum temperature for antigen-antibody reaction will depend on the chemical nature of the epitope, paratope, and the type of bonds involved in their interaction. For example, hydrogen bond formation tends to be exothermic. These bonds are more stable at lower temperature and may be more important when dealing with carbohydrate antigens.
pH
The effect of pH on the equilibrium constant of the antigen-antibody complex lies in the pH range of 6.5 and 8.4. Below pH 6.5 and above pH 8.4, the antigen-antibody reaction is strongly inhibited. At pH 5.0 or 9.5, the equilibrium constant is 100-fold lower than at pH 6.5 - 7.0. Under extreme pH conditions, antibodies may undergo conformational changes that can destroy the complementarity with the antigen.
Ionic Strength
Effect of ionic strength on antigen-antibody reaction is particularly important in blood group serology. Here the reaction is significantly influenced by sodium and chloride ions. For example, in normal saline solution, Na+ and Cl− cluster around the complex and partially neutralize charges, potentially interfering with antibody binding to antigen. This could be problematic when low-affinity antibodies are used. It is well known that, when exposed to very low ionic strengths, γ-globulins aggregate and form reversible complexes with lipoproteins of red blood cells, leading to their sedimentation.
Continue learning about antibodies with our article on Antibody Generation.
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?