Alkali Metal Silica Gels from SiGNa
Introduction
Alkali metals have long been used in synthetic chemistry as reducing agents, but their pyrophoric nature has often prevented their use in larger-scale reactions. Alkali metals react violently with water, produce copious amounts of hydrogen gas that can ignite, and result in strongly alkaline solutions. The neutral metals usually need to be stored under vacuum or in an inert liquid to protect them before use. Typically, synthetic chemists have used these metals in reactions as dispersions, as solutions in liquid ammonia, or on inert supports to avoid some of these concerns. However, a need still exists to have alkali metals and alloys available in a form that is easily handled without a significant loss in metal reactivity.
SiGNa Chemistry has recently developed a technology1 for encapsulating alkali metals into nano-structured porous oxides to create stable, free flowing powders with varying reactivities (based on the intended application). These novel materials reduce the hazards associated with the handling of alkali metals while retaining the reducing power of the parent metal or alloy. Sodium or sodium-potassium alloys in silica gel (Na-SG, Na2K-SG, and K2Na-SG) are free-flowing solids that have demonstrated applications in desulfurization, Birch reduction, ester reduction, and a variety of amine deprotections at room temperature and atmospheric pressure, thus eliminating a need for high pressure and high temperature systems.
SiGNa’s alkali silica gels are classified into three categories. Stage 0 powders are air-sensitive, but can easily be used in continuous-flow applications. Stage I powders are non-pyrophoric and dry air-stable; they can be stored for months without any change in reducing capacity. Stage II powders are easily handled in an open ambient environment, but readily react with water to produce stoichiometric yields of pure hydrogen gas. Stage II powders function well as a hydrogen source or drying agent. SiGNa’s research advances with alkali silica gels made them a winner of the 2008 Presidential Green Chemistry Challenge Award.
Desulfurizations
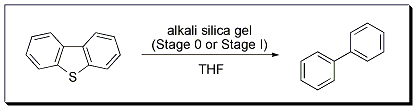
Normally, alkali metal-mediated desulfurizations in hydrocarbon solvents require high temperatures. However, the desulfurization of dibenzothiophene in THF to form biphenyl using Stage 0 or Stage I materials in continuous or batch processes can be accomplished at room temperature. The methodology was also successfully applied to the more difficult desulfurizations of 4,6-dimethyldibenzothiophene and diphenyl sulfide.
Birch Reduction
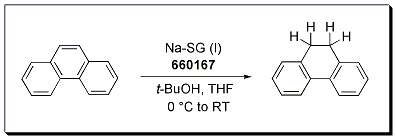
The Birch reduction of phenanthrene occurs cleanly to afford 9,10-dihydrophenanthrene in 89% yield using Na-SG (Stage I) as the reducing agent. The reaction is carried out in THF and utilizes t-BuOH as the proton source. A Birch reduction under SiGNa conditions requires no liquid ammonia, no cryogenic temperatures, and no pyrophoric reagents.
Bouveault-Blanc reduction
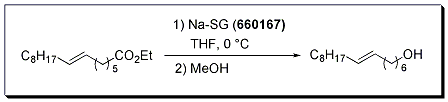
Improved conditions for the Bouveault-Blanc reduction of esters to alcohols have been developed using sodium in silica gel (Na-SG). Primary alcohols were prepared in excellent yield from a variety of aliphatic esters under mild reaction conditions. This chemistry is a safer alternative to the classic Bouveault-Blanc reduction, and avoids reaction workup issues associated with the formation of aluminum salts in conventional lithium aluminum hydride reductions. Interestingly, isolated alkenes were left intact under the standard reaction conditions.
Reductive Deprotection
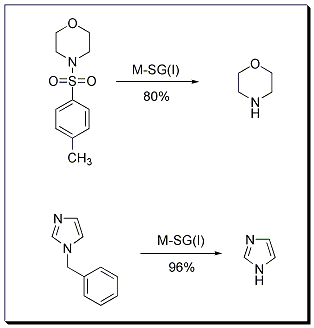
Reductive cleavage of a C-N bond is an important synthetic transformation that is frequently applied in functional group transformations and in deprotection reactions such as debenzylation and detosylation. SiGNa has recently reported a novel method to cleave toluenesulfonamides to amines using M-SG. Specifically, M-SG (Stage I) where M is Na or Na2K has been used to achieve this transformation. Analogously, debenzylation is achieved using similar reaction conditions.
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?