Create a 3D Kidney Nephrotoxicity Model on the AIM Biotech 3D Cell Culture Chip
Kidney Model for Toxicity and Clearance
The SA7K cell line (MTOX1030) is a pseudo-immortalized human renal proximal tubule epithelial cell (RPTEC) line that was developed from primary cells using the CompoZr® Zinc Finger Nuclease technology. The cells were engineered to avoid cellular senescence, and thus enable extended population doublings. SA7K cells exhibit normal epithelial morphology, express characteristic proximal tubule markers (e.g., CD-13 and α-GST), and perform typical proximal tubule functions, including albumin uptake, γ-glutamyl transpeptidase activity, increased secretion of cAMP in response to parathyroid hormone, and functional activity of both uptake and efflux transporters.
SA7K cells were loaded onto the AIM chip on Day 0. Cells were analyzed by immunofluorescence for their ability to accurately polarize on Day 6. (Figures 1-2).
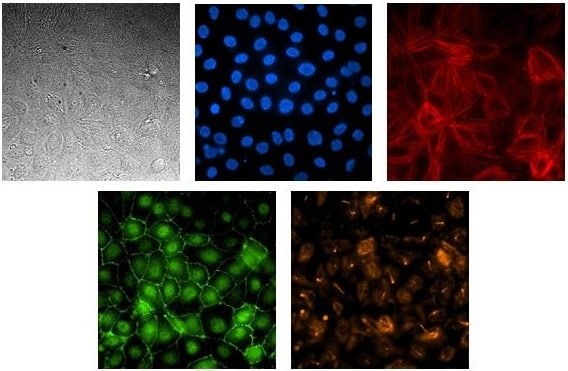
Figure 1. Renal proximal tubule epithelial cell line SA7K form a polarized, tight barrier on the AIM Biotech chip.Cells were loaded onto the AIM 3D cell culture chip and allowed to grow for six days without agitation. Cells in the chip were imaged by standard microscopy techniques. The images show RPTEC in bright-field microscopy stained for nuclear (Hoescht, blue), F-actin (phalloidin, red), ZO-1 (green), and acetylated-tubulin (orange).
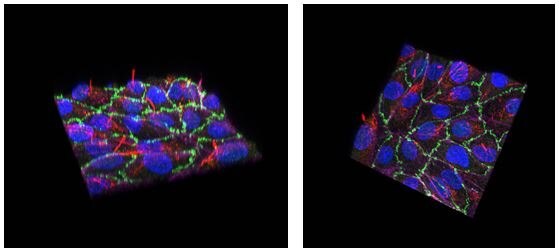
Figure 2. Renal proximal tubule epithelial cell line SA7K forms a polarized, tight barrier on the AIM Biotech chip.Cells were loaded onto the AIM 3D cell culture chip and allowed to grow for six days without agitation. Cells in the chip were imaged by confocal microscopy. The images show an overlay of RPTECs stained for nuclear (Hoescht, blue), F-actin (phalloidin, magenta), ZO-1 (green), and acetylated-tubulin (red). The staining for acetylated tubulin is on the apical side of the cells, indicating the cells are accurately polarized in the AIM chip.
Quantifying Barrier Integrity with a Permeability Assay
A permeability assay is the most common method to quantify the integrity of a barrier model. In this permeability assay, dyes of varying sizes are added to an established barrier and leakage of the dye through the barrier is measured. Comparing the amount of dye in the cell channel to the adjacent channel will give a calculated value for apparent permeability, which indicates the tightness of the barrier.
RPTEC SA7K cells were loaded onto the AIM chip on Day 0 and allowed to form a tubule. On Day 14, dyes were loaded into the lumen of the tubule formed in cell channel. The dye was incubated in the lumen for 10 minutes. Images of the channel with the cells and dye and the adjacent empty channel were collected at time 0 and after 10 minutes. The movement of the dye, measured by the intensity of light emitted from the dye in each channel, was measured at time 0 and after 10 minutes. The measured intensity values were input into the equation for apparent permeability, and a value for apparent permeability was calculated for each dye (Figure 3).

Figure 3. Permeability assay for RPTEC SA7K on the AIM chip.After 14 days incubation of RPTEC SA7K cells on the AIM chip, Lucifer yellow (MW 457.25) and TRITC-labeled dextran (MW 3000) were added to the lumen of the RPTEC tubule in the cell channel (represented by green and red bars). Images were collected at the start and after 10 minutes of incubation of the dye in the cell channel. Leakage of the dyes through the RPTEC barrier was measured by comparing the intensities of the light in the adjacent channel (identified by the yellow box in the first panel) at time 0 and 10 minutes. Note that the yellow box identifying the adjacent channel in the first panel is for illustration only and does not accurately represent the area of the box used for the calculating apparent permeability.
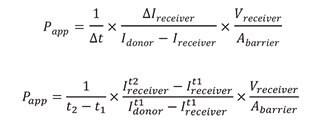
Using the equation below, values of the intensities of the cell channel (Idonor) and the adjacent channel (Ireceiver) were input with measurements for the area and volume of the chip to determine the apparent permeability (Papp).
The measured intensities and calculated Papp values for both Lucifer yellow and TRITC in the RPTEC SA7K AIM chip platform are listed in Table 1. Both Papp values are extremely low, quantitatively showing the tight integrity of the barrier formed by RPTEC SA7K cells plated on the AIM chip with no agitation or shear stress.
Conclusions
The design of the AIM Biotech chip enables easy setup of 3D microphysiological systems. The inlets for injecting hydrogel and cells are large, making it easy to load and culture cells in the platform. The growth of a complete, tight tubule structure is necessary to develop a kidney clearance model. To successfully make a leak-proof cell barrier, cells need to be distributed along the entire length of the channel. The design of the AIM 3D cell culture chip and optimized culture protocol produces an even distribution of cells throughout the entire channel, resulting in complete tubule formation. The chip’s design and polymer construction enable sampling of both apical and basolateral compartments and for generating high-resolution phase and fluorescent images.
The RPTEC SA7K cells formed a tubule structure when cultured in the AIM 3D cell culture chip. ZO-1, a tight junction protein, was detected at the cell-to-cell borders, indicating the formation of a tight barrier. The staining of primary cilium, which is located at the brush border and is oriented toward the lumen, was used to confirm accurate polarization of the cells within the tubule. The fluorescent compounds TRITC-labeled dextran (MW 3,000) and Lucifer yellow (MW 457.25) were injected into the lumen of the tubule to determine the integrity of the barrier by measuring leakage of the compounds through the barrier and into the gel channel. Papp values of 4.75 x 10-7 cm/s and 2.09 x 10-6 cm/s were determined for TRITC-dextran and Lucifer yellow, respectively, which indicate the formation of a tight barrier. Successful formation of tubule with tight barrier allows for the potential development of a kidney clearance assay in the AIM 3D cell culture chip.
About AIM Biotech
AIM Biotech offers a modular platform to co-culture multiple cell types in discrete 3D and 2D channels. Organotypic assays with animal model-like complexities using human cells have been developed (e.g., immune checkpoint, T-cell killing, angiogenesis, metastasis, cell migration, blood-brain barrier, etc.) for research, drug discovery & diagnostics.
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?