Enzymatic Assay and ATP Sensitivity Test of Luciferase
1. Objective
To standardize a procedure to determine the enzymatic activity of luciferase and/or to determine the ATP detection limit of luciferase.
2. Scope
This scope of this procedure applies to products that have a specification for enzymatic activity of luciferase and/or a specification for ATP sensitivity.
3. Definitions
3.1 ATP = Adenosine 5’-triphosphate
3.2 AMP = Adenosine 5’-monophosphate
Purified water = water from a deionizing system, resistivity ~18MΩ•cm @ 25 ºC
4. Discussion
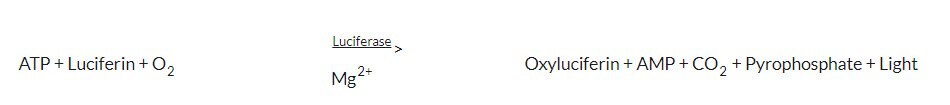
5. Responsibilities
Analytical services laboratory personnel should follow this protocol as written.
6. Safety
Refer to the Safety Data Sheet (SDS) for hazards and appropriate handling precautions.
7. Procedure
7.1 Enzymatic Assay of Luciferase
7.1.1 CONDITIONS: T = 25 ºC
7.1.2 METHOD: Determination of light emission measured by a luminometer
7.1.3 REAGENTS
7.1.3.1 1 M Glycine-Tris Buffer, 10mM EDTA, 100 mM magnesium sulfate, pH 7.6 at 25 ºC (Enzyme Diluent)
Prepare a 75.1 mg/mL solution of glycine (Product No. G7126) 0.37 mg/mL of EDTA (Product No. ED2SS) and 24.7 mg/mL of magnesium sulfate (Product No. M1880) in purified water. Adjust pH to 7.6 at 25 ºC with Trizma (Product No. T1503).
7.1.3.2 50 mM glycine, 0.15 mM luciferin, 1 mM Tris, pH 7.6, 5 mM MgSO4, 0.55 mM EDTA, 0.1% (w/v) BSA, 0.1% (w/v) sodium azide (firefly). Prepare vial of Firefly Diluent (Product No. F3766) according to vial instructions. Store on ice.
7.1.3.3 1 mM ATP (Conc ATP)
Prepare a 0.55 mg/mL solution of ATP (Product No. A2383) in purified water.
7.1.3.4 0.01 mM ATP (ATP)
Prepare a 1 to 100 dilution of Reagent 7.1.3.3 in purified water.
7.1.3.5 Luciferase (Enzyme)
7.1.3.5.1. Immediately before use, prepare a solution containing 3,000,000 – 4,000,000 units/mL of Luciferase in cold reagent 7.1.3.1 (Enzyme Diluent). When dissolving enzyme use minimal agitation. For best results add enzyme diluent to enzyme and place on ice for minimum 5 minutes without any agitation. Vortexing and excessive shaking/swirling causes the enzyme to clump and not dissolve. If clumping is observed weigh fresh enzyme.
7.1.4. TEST METHOD ENZYMATIC ASSAY OF LUCIFERASE
7.1.4.1 Luminometer Settings:
Delay Time: 0 sec
Integrate Time: 4 sec
Number of Replicates: 1
Mode: STD
7.1.4.2 Standardizing the instrument: Wipe the outside of the 14C standard vial. Place the 14C standard vial in the cell compartment. Press the GO button and record the number (intensity) at end of the read time. Take five readings. The average of five intensity readings should be 48–52.
7.1.4.3 Prepare reaction cocktail by adding 0.010 mL of reagent 7.1.3.4 (ATP) to 0.990 mL of reagent 7.1.3.2 (firefly)
7.1.4.4 Pipette (in milliliters) the following reagents into suitable test tubes:
Allow to equilibrate to 25 ºC for 5 min.
7.1.4.5 Then add the following using gel loading pipette tips:
Immediately mix test tube by tapping and place in cell compartment. Press the GO button and record the number (intensity) at the end of the read time. Reading must happen quickly after addition of enzyme (within 3-5 seconds).
7.1.4.6 Repeat steps 7.1.4.4 through 7.1.4.5 for 5 additional readings.
7.1.5 CALCULATIONS
7.1.5.1 Average the six emission intensities.
DF = Dilution factor
0.001 = Volume (in milliliters) of enzyme used
7.1.6 UNIT DEFINITION
One light unit produces a biometer peak height equivalent to 0.02 µCi of 14C in PPO/POPOP cocktail. Light units measured in 50 µL assay mixture containing 5 pmol ATP and 7.5 nmol luciferin in Tris-glycine buffer, pH 7.6, at 25 °C.
7.1.7 FINAL ASSAY CONCENTRATION
In a 0.051 ml reaction mix, the final concentrations are 0.15 mM Luciferin, 49 mM glycine, 0.98 mM Tris, 7 mM magnesium sulfate, 0.7 mM EDTA, 0.1% (w/v) BSA, 0.1% (w/v) Sodium Azide, 100 nM ATP, and 3,000 – 4,000 units Luciferase.
7.2 ATP Sensitivity Test of Luciferase
7.2.1 CONDITIONS: T = 25 ºC
7.2.2 METHOD: Determination of light emission measured by a luminometer
7.2.3 REAGENTS
7.2.3.1 0.02 mM ATP
Prepare a 1 to 50 dilution of Reagent 7.1.3.3 in purified water.
7.2.3.2 0.002 mM ATP (ATP2)
Prepare a 1 to 10 dilution of Reagent 7.2.3.1 in purified water.
7.2.3.3 Luciferase (Enzyme)
7.2.3.3.1. Immediately before use, prepare a solution containing 0.2 mg P/mL of Luciferase in cold reagent 7.1.3.1 (Enzyme Diluent).
7.2.3.3.2. Note: When dissolving enzyme use minimal agitation. For best results add enzyme diluent to enzyme and place on ice for minimum 5 minutes without any agitation. Vortexing and excessive shaking/swirling causes the enzyme to clump up and not dissolve. If clumping is observed weigh fresh enzyme.
7.2.4. TEST METHOD FOR ATP SENSITIVITY OF LUCIFERASE
7.2.4.1 If necessary, follow steps 7.1.4.1 and 7.1.4.2 to set and standardize the luminometer.
7.2.4.2 Prepare reaction cocktail by adding 0.010 mL of reagent 7.2.3.2 (ATP2) to 0.990 mL of reagent 7.1.3.2 (firefly).
7.2.4.3 Pipette ( in milliliters) the following reagents into suitable test tubes:
Allow to equilibrate to 25 ºC for 5 min.
7.2.4.4 Then add the following using gel loading pipette tips:
Immediately mix test tube by tapping and place in cell compartment. Press the GO button and record the number (intensity) at the end of the read time. Reading must happen quickly after addition of enzyme (within 3-5 seconds).
7.2.4.5 Repeat steps 7.2.4.3 through 7.2.4.4 for 5 additional readings.
7.2.5 CALCULATIONS
7.2.5.1 Average all 6 Emission Intensities.
7.2.5.2 The ATP Sensitivity Test is a measure of how much ATP yields 1 count on the luminometer. Each reaction mixture contains one picomole of ATP and yields a certain number of counts on the luminometer. The calculation is 1 picomole divided by the number of counts that resulted from that trial. The result is expressed in femtomoles (picomoles *1000), and is called the ATP detection limit.
7.2.6 UNIT DEFINITION
NA
7.2.7 FINAL ASSAY CONTENTRATION
In a 0.051 mL reaction, the final concentrations are 0.15 mM luciferin, 49 mM glycine, 0.98 mM Tris, 7 mM magnesium sulfate, 0.7 mM EDTA, 0.1% (w/v) BSA, 0.1% (w/v) sodium azide, 20 nM ATP, and 0.2 µg protein of Luciferase.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?