LC/MS Analysis of Nucleosides on Ascentis® Express F5
Materialien
Analytische Säule
Komponente der mobilen Phase
Standard
Guanosin-5′-Monophosphat Dinatriumsalz Hydrat
Pharmaceutical Secondary Standard; Certified Reference MaterialCONDITIONS
column
Ascentis Express F5, 15 cm x 2.1 mm I.D., 2.7 μm particles, 90Å (53571-U)
mobile phase
[A] 5 mM ammonium acetate, pH 5.5 with acidic acid in water; [B] Methanol
gradient
hold at 0% for 6 min; to 50% B in 4 min; hold at 50% B for 4 min; to 0% B in 0.1 min; to hold at 0% B for 5.9 min
flow rate
0.3 mL/min
pressure
5017-9367 psi
column temp.
15 °C
detector
MS, ESI (-) QTOF, EIC
injection
5 μL
sample
100 μg/mL of 12 nucleosides except N6-methyladenosine in 99:1 10mM ammonium acetate pH 5.5 in water : methanol
Beschreibung
Allgemeine Beschreibung
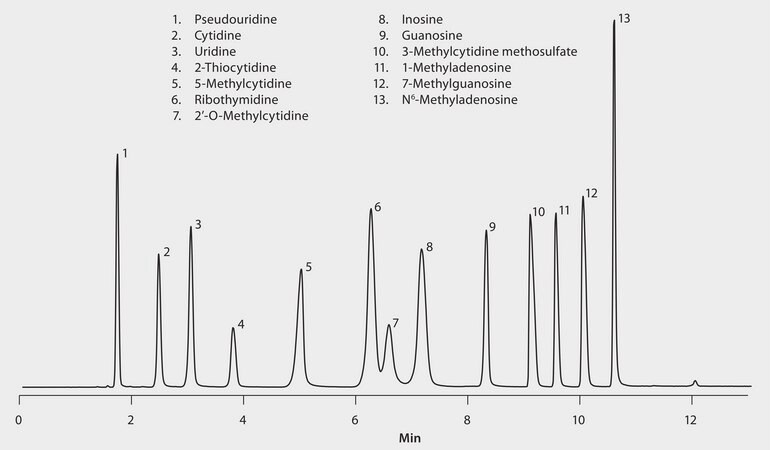
Detector: MS, ESI (-) QTOF, EIC, (Pseudouridine m/z 243 and 153; Cytidine m/z 242 and 109; Uridine m/z 243 and 111; 2-Thiocytidine m/z 258 and 126; Guanosine m/z 282 and 150; Inosine m/z 267 and 135; Ribothymidine m/z 257 and 125; 1-Methyladenosine m/z 280 and 148; 2′-O-Methyladenosine m/z 256, 213 and 108; 5-Methylcytidine m/z 256 and 124; 7-Methylguanosine m/z 124 and 164; 3-Methylcytidine methosulfate m/z 256 and 124; N6-Methyladenosine m/z 280 and 148.
Hinweis zur Analyse
Nucleosides are structural subunits of nucleic acids, the macromolecules that convey genetic information in living cells. They contain a molecule of sugar (pentose) bonded to a nitrogen-containing organic ring compound. The nitrogen-containing compound is either a pyrimidine (cytosine, thymine, or uracil) or a purine (adenine or guanine). Nucleosides, derivatives and / or analogues can be used in many different industries and applications as in agrochemistry (herbicides, fungicides and insecticides), therapeutic drugs including a range of antiviral (HIV, hepatitis C virus and herpes simplex) and even antibiotics. This application demonstrates reverse phase interactions with mobile phases that are shown to work well in mass spectrometry conditions.
Sonstige Hinweise
1-methyladenosine undergoes a Dimroth rearrangement to yield N6-methyladenosine in the standard solution mix.
Rechtliche Hinweise
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany