442232-U
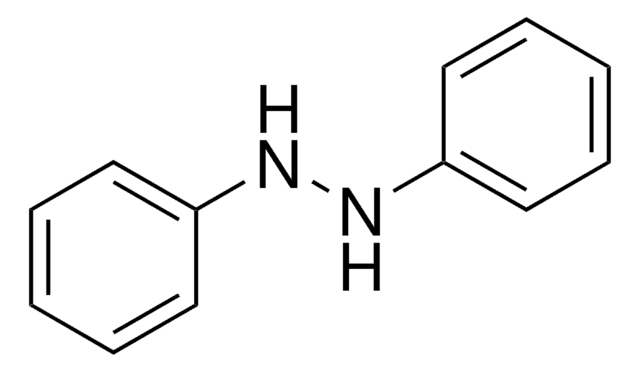
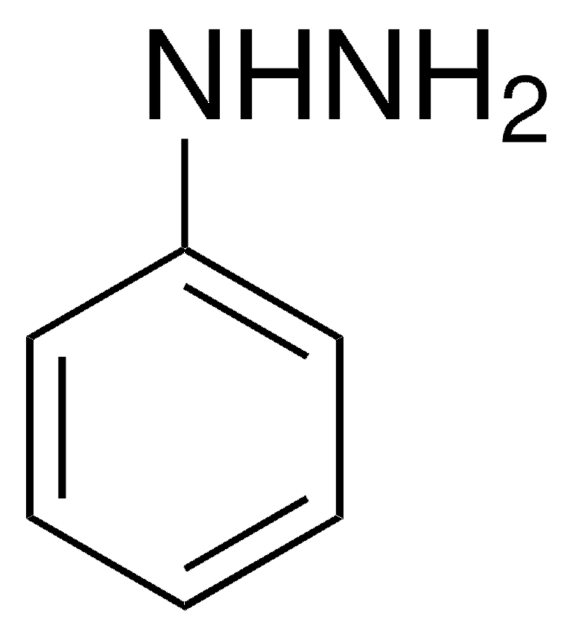
1,2-Diphenylhydrazin
analytical standard, ampule of 100 mg
Synonym(e):
Hydrazobenzol, N,N′-Diphenylhydrazin, N,N′-Bianilin, NSC 3510
About This Item
Empfohlene Produkte
Qualität
analytical standard
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
ampule of 100 mg
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
mp (Schmelzpunkt)
123-126 °C (lit.)
Anwendung(en)
cleaning products
cosmetics
environmental
food and beverages
personal care
Format
neat
Lagertemp.
2-30°C
SMILES String
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
InChIKey
YBQZXXMEJHZYMB-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Insertion reactions with organometallic tantalum complexes
- Reduction reactions catalyzed by titanium(III) trichloride yielding amines
- Studying the mechanism of hydrazobenzene rearrangement
- Reaction with N-heterocyclic stable silylene
- Synthesis of dimanganese amide hydrazide cluster complexes
- Iron-mediated hydrazine reductions yielding iron arylimide cubanes
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Lagerklassenschlüssel
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.