86320
Weinsteinsäure
≥97.0%
Synonym(e):
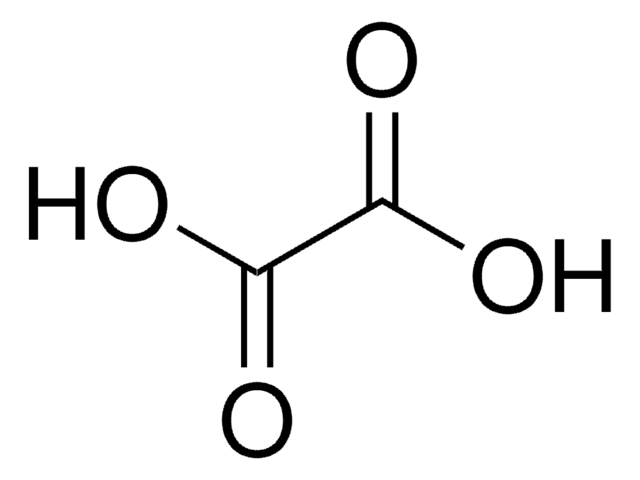
Hydroxymalonsäure
About This Item
Empfohlene Produkte
Assay
≥97.0%
Form
powder
mp (Schmelzpunkt)
158-160 °C (dec.) (lit.)
Lagertemp.
2-8°C
SMILES String
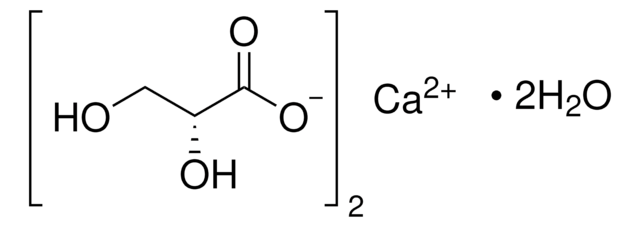
OC(C(O)=O)C(O)=O
InChI
1S/C3H4O5/c4-1(2(5)6)3(7)8/h1,4H,(H,5,6)(H,7,8)
InChIKey
ROBFUDYVXSDBQM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Polymer synthesis for enhanced thermal conductivity: Tartronic acid is used to exploit enzyme reactions in polymer synthesis, significantly increasing the thermal conductivity of materials, which is pivotal in manufacturing and material science applications (Nan et al., 2023).
- Advances in green chemical treatments: This acid plays a role in the electro-oxidation pathways for treating glycerol waste, contributing to sustainable chemical processes and green chemistry applications, which are essential for reducing environmental impact (Cheng et al., 2021).
- Development in biodiesel by-products treatment: Tartronic acid is also involved in kinetic studies for the electrochemical conversion of glycerol, a by-product of biodiesel production, highlighting its role in renewable energy and waste valorization (Pérès et al., 2020).
- Base-free oxidation reactions: It aids in the development of base-free conditions for glycerol to glyceraldehyde oxidation reactions over platinum-based catalysts, offering advancements in catalysis and organic synthesis processes (Capron et al., 2019).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.