MC0SP03FS1
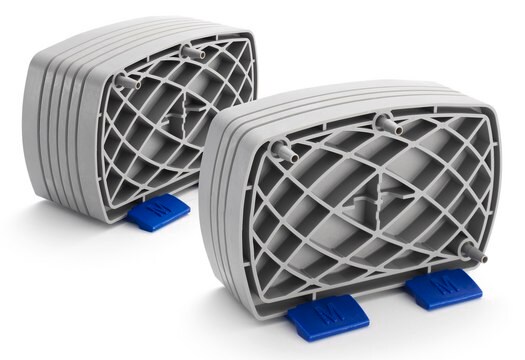
Millistak+® HC Pro Pod Tiefenfilter
C0SP, filtration area 0.33 m2, hydrophilic
Synonym(e):
Millistak+ HC Pro Pod Depth Filter, C0SP media series, 0.33 m 2 surface area
About This Item
Empfohlene Produkte
Materialien
acrylic
polypropylene
silica filter aid
Qualitätsniveau
Beschreibung
Primary clarification directly out of the bioreactor
Produktlinie
EMPROVE® Filter
Leistungsmerkmale
hydrophilic
Hersteller/Markenname
Millistak+®
Parameter
30 psid max. differential pressure (2.1 bar) at 25 °C (Reverse)
30 psid max. differential pressure (2.1 bar) at 80 °C (Forward)
50 psid max. inlet pressure (3.5 bar) at 80 °C
Höhe
12.5 in. (32 cm)
317 mm
Länge
24.2 in. (62 cm)
depth
3.2 in. (8.1 cm)
Filtrationsfläche
0.33 m2
Verunreinigungen
<0.25 EU/mL bacterial endotoxins (LAL test, Aqueous extraction)
Aufnahme
sample type liquid
Leitfähigkeit
18.9-41.5 μS/cm at 25 °C (product water)
Filterqualität
C0SP
Allgemeine Beschreibung
Anwendung
Leistungsmerkmale und Vorteile
- Reduce TOC extractables and pre-use flush volume requirements by 50%
- No beta glucans to interfere with limulous amoebocyte lysate (LAL) testing for bacterial endotoxins
- Lot to lot consistency for successful development and implementation of robust clarification processes
Depth filter media formulation & design
- Provide as much as two times the filtration capacity with equivalent filter retention properties over commercial DE-based benchmarks
- Improved HCP impurity clearance
Disposable Pod device
- Flexible, modular format offers scalability from 5 to 12,000 liters or more
- Robust device format; easy to use and set up
Spezifikationen
Angaben zur Herstellung
Integrity is maintained after 1 autoclave cycle of 60 min @ 123 ºC. Filtration performance may be impacted post autoclave. Recommended for post use decontamination only.
Hinweis zur Analyse
Rechtliche Hinweise
Sie haben nicht das passende Produkt gefunden?
Probieren Sie unser Produkt-Auswahlhilfe. aus.
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.