MABN687
Anti-β-amyloid fibril-specific, clone B10, AP Antibody
clone B10, from camel, alkaline phosphatase conjugate
Synonym(e):
Amyloid beta A4 protein, ABPP, APPI, APP, Alzheimer disease amyloid protein, Cerebral vascular amyloid peptide, CVAP, PreA4, Protease nexin-II, PN-II, N-APP2.Soluble APP-alpha, S-APP-alpha, Soluble APP-beta, S-APP-beta, C99, Beta-amyloid protein 42, Beta
About This Item
Empfohlene Produkte
Biologische Quelle
camel
Qualitätsniveau
Konjugat
alkaline phosphatase conjugate
Antikörperform
purified immunoglobulin
Antikörper-Produkttyp
primary antibodies
Klon
B10, monoclonal
Speziesreaktivität
human
Methode(n)
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
Isotyp
IgG
NCBI-Hinterlegungsnummer
UniProt-Hinterlegungsnummer
Versandbedingung
dry ice
Posttranslationale Modifikation Target
unmodified
Angaben zum Gen
human ... APP(351)
Allgemeine Beschreibung
Immunogen
Anwendung
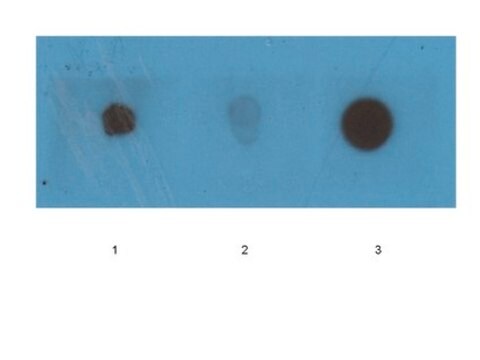
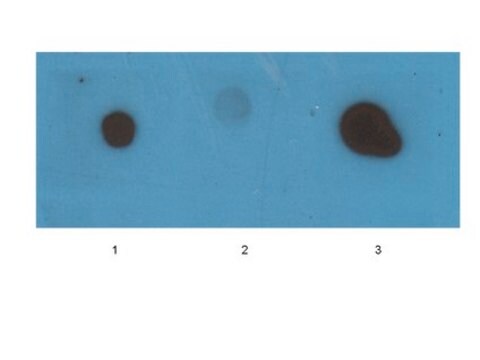
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in synthetic Aβ (1–40) peptide (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in chemically modified fibrils (Haupt, C., et al. (2011). J. Mole. Biol. 405:341-348).
Elisa Analysis: A representative lot detected β-amyloid fibril-specific in N-biotinylated Aβ (1–40) conformers (disaggregated peptide, oligomers, or fibrils) (Morgado, I., et al. (2012). PNAS. 109(31):12503-12508).
Immunohistochemistry Analysis: A representative lot detected β-amyloid fibril-specific in Hippocampal sections from Alzheimer brain tissue (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
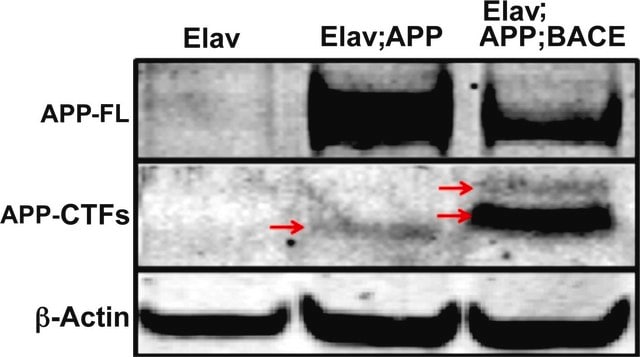
Immunoprecipitation Analysis: A representative lot detected β-amyloid fibril-specific in native soluble and dispersible fractions from the brain lysates (Upadhaya, A.R., et al. (2014). BRAIN. 1-17).
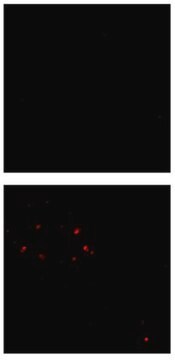
Immunofluorescence Analysis: A representative lot detected β-amyloid fibril-specific in cell culture-derived amyloid plaques (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Qualität
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected β-amyloid fibril-specific in human Alzheimer′s brain tissue.
Physikalische Form
Note: This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
Sonstige Hinweise
Not finding the right product?
Try our Produkt-Auswahlhilfe.
Lagerklassenschlüssel
12 - Non Combustible Liquids
WGK
nwg
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.