W242705
Ethylbuttersäure
≥98%, FCC, FG
Synonym(e):
Ethyl-butyrat
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
FG
Halal
Kosher
Agentur
meets purity specifications of JECFA
Einhaltung gesetzlicher Vorschriften
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 182.60
Dampfdichte
4 (vs air)
Dampfdruck
15.5 mmHg ( 25 °C)
Assay
≥98%
Selbstzündungstemp.
865 °F
Brechungsindex
n20/D 1.392 (lit.)
bp
120 °C (lit.)
mp (Schmelzpunkt)
−93 °C (lit.)
Dichte
0.875 g/mL at 25 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Organoleptisch
ethereal; fruity; pineapple; sweet
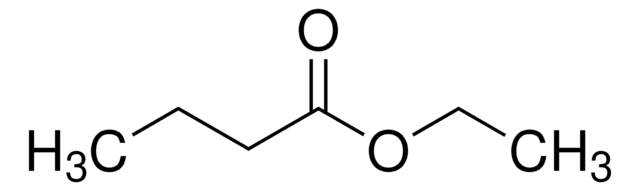
SMILES String
CCCC(=O)OCC
InChI
1S/C6H12O2/c1-3-5-6(7)8-4-2/h3-5H2,1-2H3
InChIKey
OBNCKNCVKJNDBV-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Timolol transport from microemulsions trapped in HEMA gels.: This article examines the microemulsion systems for drug delivery, where ethyl butyrate could play a role in solubilizing components (Li CC et al., 2007).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Flam. Liq. 3
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 1
Flammpunkt (°F)
78.8 °F - closed cup
Flammpunkt (°C)
26 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Protokolle
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.