80645
Piperidine solution
suitable for peptide synthesis, 20% in DMF
Synonym(s):
Azacyclohexane, Cyclopentimine, Hexahydropyridine, Pentamethyleneimine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
Beilstein:
1421082
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.31
Recommended Products
form
liquid
Quality Level
concentration
20% in DMF
impurities
≤0.1% water
refractive index
n20/D 1.434
density
0.930 g/mL at 20 °C
application(s)
peptide synthesis
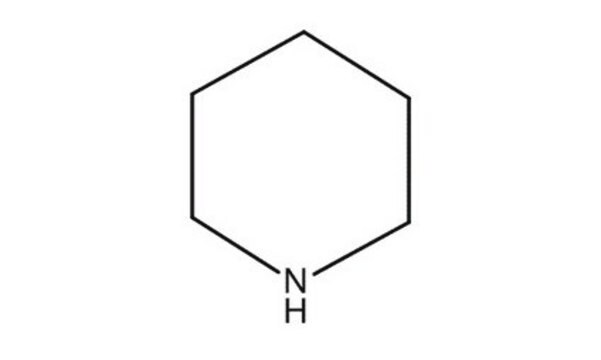
SMILES string
C1CCNCC1
InChI
1S/C5H11N/c1-2-4-6-5-3-1/h6H,1-5H2
InChI key
NQRYJNQNLNOLGT-UHFFFAOYSA-N
Application
- Targeted potent antimicrobial and antitumor oxygen-heterocyclic-based pyran analogues: synthesis and computational studies.: This research highlights the application of piperidine solution in the synthesis of novel pyran analogues, demonstrating its utility in enhancing antimicrobial and antitumor properties through advanced chemical synthesis and computational modeling techniques (El-Wahab et al., 2024).
- Heterocyclic Amine-Induced Feeding Deterrence and Antennal Response of Honey Bees.: Utilizing piperidine solution, this study examines the impact of heterocyclic amines on honey bees, providing valuable insights into the chemical ecology of plant-insect interactions and potential strategies for pest control in agriculture (Larson et al., 2021).
- Effects of Desilication in NaOH/Piperidine Medium and Phosphorus Modification on the Catalytic Activity of HZSM-5 Catalyst in Methanol to Propylene Conversion.: This study uses piperidine solutions to modify the surface properties of HZSM-5 catalysts, significantly enhancing their performance in methanol to propylene conversion processes, highlighting the application in industrial catalysis (Safaei et al., 2021).
- MWW-Type Titanosilicate Synthesized by Simply Treating ERB-P Zeolite with Acidic H(2)TiF(6) and Its Catalytic Performance in a Liquid Epoxidation of 1-Hexene with H(2)O(2).: This research utilizes piperidine solution in the synthesis of novel titanosilicate materials, exploring its effectiveness in the catalytic epoxidation of hexene, demonstrating the potential of piperidine in facilitating advanced material synthesis (Guo et al., 2020).
Other Notes
Sales restrictions may apply
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Repr. 1B - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carmine Pasquale Cerrato et al.
Journal of materials chemistry. B, 8(47), 10825-10836 (2020-11-12)
Cell-penetrating peptides are a promising therapeutic strategy for a wide variety of degenerative diseases, ageing, and cancer. Among the multitude of cell-penetrating peptides, PepFect14 has been preferentially used in our laboratory for oligonucleotide delivery into cells and in vivo mouse
Xuwang Chen et al.
Bioorganic & medicinal chemistry, 20(12), 3856-3864 (2012-05-18)
A novel series of piperidine-linked amino-triazine derivatives were designed, synthesized and evaluated for in vitro anti-HIV activity as non-nucleoside reverse transcriptase inhibitors on the basis of our previous work. Screening results indicated that most compounds showed excellent activity against wild-type
Total synthesis of (-)-kopsinine by an asymmetric one-pot [n+2+3] cyclization.
Shingo Harada et al.
Chemistry, an Asian journal, 7(10), 2196-2198 (2012-08-22)
P Ricardo Girling et al.
Organic & biomolecular chemistry, 9(9), 3105-3121 (2011-03-11)
A review into the aza-Diels-Alder reaction, mainly concentrating on literature examples that form piperidin-4-ones from the reaction of imines and electron rich dienes or enones, either through a Lewis acidic/Brønsted acid approach or through the use of an organocatalyst. This
Elisabeth T Hennessy et al.
Science (New York, N.Y.), 340(6132), 591-595 (2013-05-04)
The manipulation of traditionally unreactive functional groups is of paramount importance in modern chemical synthesis. We have developed an iron-dipyrrinato catalyst that leverages the reactivity of iron-borne metal-ligand multiple bonds to promote the direct amination of aliphatic C-H bonds. Exposure
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service