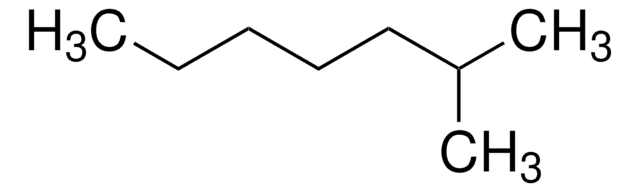
About This Item
Recommended Products
vapor density
3 (vs air)
vapor pressure
6.77 psi ( 37.7 °C)
Assay
≥99%
form
liquid
autoignition temp.
583 °F
expl. lim.
7 %
refractive index
n20/D 1.371 (lit.)
bp
62 °C (lit.)
mp
−154 °C (lit.)
density
0.653 g/mL at 25 °C (lit.)
SMILES string
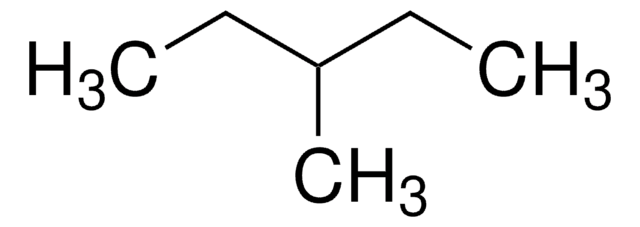
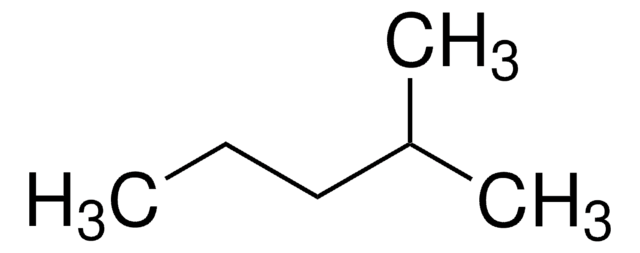
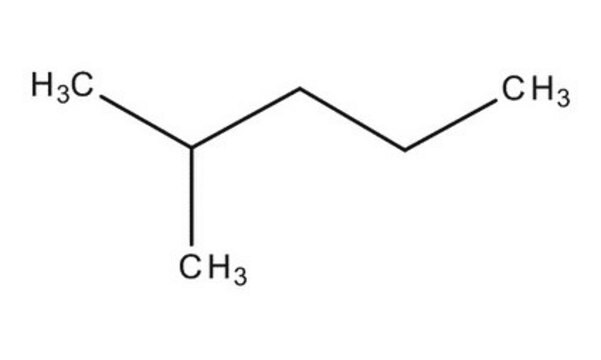
CCCC(C)C
InChI
1S/C6H14/c1-4-5-6(2)3/h6H,4-5H2,1-3H3
InChI key
AFABGHUZZDYHJO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Xylene; Nonane; Decane; 1,2,4-Trimethylbenzene; Butylcyclohexane; Naphthalene
-1,3-Dimethylcyclopentane; 1,1-Dimethylcyclopentane; 2,2,3-Trimethylpentane; 2,2-Dimethylbutane; 2,2-Dimethylhexane; 2,2-Dimethylpentane; 2,3-Dimethylbutane; 2,3-Dimethylhexane; 2,4-Dimethylheptane; 2,4-Dimethylpentane; 2,5-Dimethylheptane; 2-Methylhexane; 2-Methylpentane; 3,3-Dimethylpentane; 3,4-Dimethylhexane; 3-Ethylpentane; 3-Methyloctane; 4-Methylheptane; Ethylbenzene; Ethylcyclopentane; 2,6-Dimethylheptane; 3-Ethylheptane
Protocol for GC Analysis of Hydrocarbons in Gasoline on Petrocol® DH
-Xylene; Nonane; Propylbenzene; Mesitylene; 1,2,4-Trimethylbenzene; 1,2,3-Trimethylbenzene; 1,3-Diethylbenzene; 1,4-Dimethyl-2-ethylbenzene; 1,2-Dimethyl-4-ethylbenzene; Durene; 1,2,3,5-Tetramethylbenzene; 1,2,3,5-Tetramethylbenzene; 2-Methylnaphthalene (β)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service