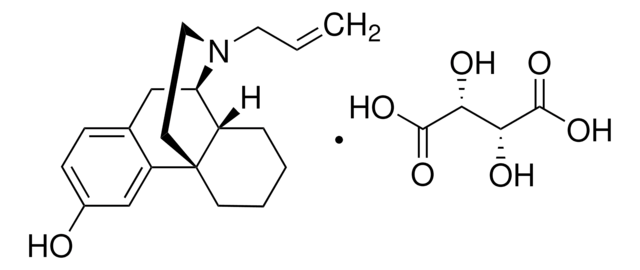
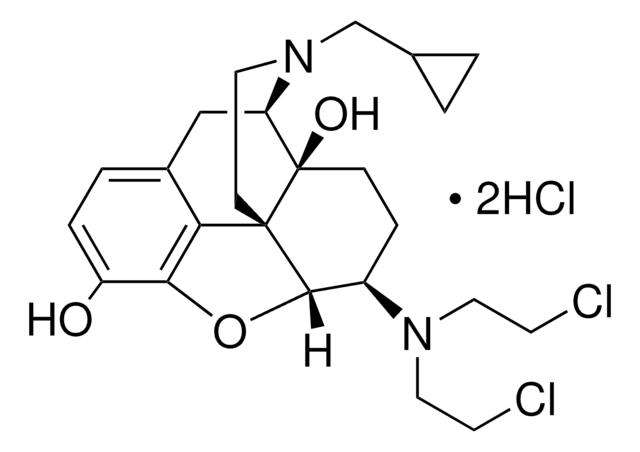
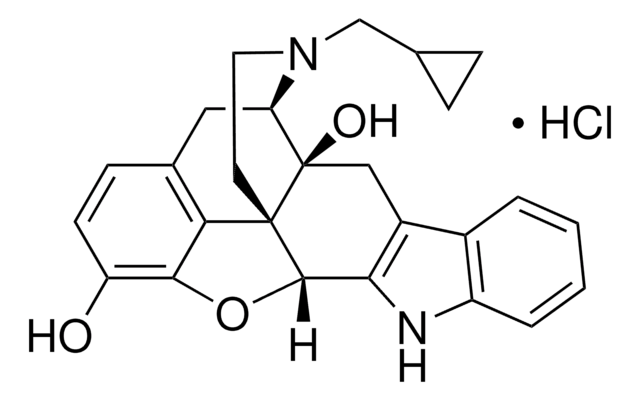
G3416
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate
solid, ≥98% (HPLC)
Sinonimo/i:
GNTI
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
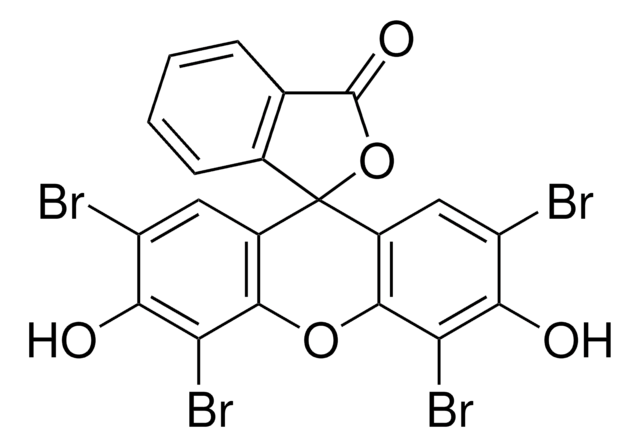
Formula empirica (notazione di Hill):
C27H29N5O3 · 2C2HF3O2 · xH2O
Numero CAS:
Peso molecolare:
699.60 (anhydrous basis)
Codice UNSPSC:
12352202
ID PubChem:
NACRES:
NA.77
Prodotti consigliati
Saggio
≥98% (HPLC)
Forma fisica
solid
Solubilità
H2O: 30 mg/mL
Temperatura di conservazione
2-8°C
Informazioni sul gene
human ... OPRK1(4986)
Applicazioni
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate (GNTI) has been used as a selective κ opioid receptor antagonist:
- to study the influence of opioid receptor types on the anti-hyperalgesic effect of dipeptidyl peptidase 4 (DPP4) inhibitors in inflammation
- to study the role of κ opioid receptor in the forebrain-dependent associative task, Whisker-Trace Eyeblink conditioning (WTEB)
- to validate its inhibitory actions on Akt kinase activities
Azioni biochim/fisiol
5′-Guanidinonaltrindole di(trifluoroacetate) (GNTI) is a 5‘-guanidine derivative and a selective κ opiate receptor antagonist. It is five-fold more potent and 500-fold more selective than norbinaltorphimine (nor-BNI) for the κ opioid receptor in smooth muscle preparations.
Caratteristiche e vantaggi
This compound is featured on the Opioid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Note legali
Sold under US Patent No. 6,500,824.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
W C Stevens et al.
Journal of medicinal chemistry, 43(14), 2759-2769 (2000-07-14)
The indole moiety in the delta-opioid antagonist, naltrindole (2, NTI), was employed as a scaffold to hold an "address" for interaction with the kappa-opioid receptor. The attachment of the address to the 5'-position of the indole moiety was based on
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific reports (2017)
R M Jones et al.
European journal of pharmacology, 396(1), 49-52 (2000-05-24)
5'-Guanidinonaltrindole (GNTI) possesses 5-fold greater opioid antagonist potency (K(e)=0.04 nM) and an order of magnitude greater selectivity (selectivity ratios >500) than the prototypical kappa-opioid receptor antagonist, norbinaltorphimine, in smooth muscle preparations. Binding and functional studies conducted on cloned human opioid
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific Reports (2017)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.