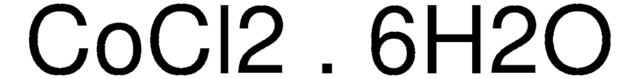60818
Cobalt(II) chloride
purum p.a., anhydrous, ≥98.0% (KT)
Sinonimo/i:
Cobalt dichloride, Cobalt muriate, Cobaltous chloride, Dichlorocobalt
About This Item
Prodotti consigliati
Tensione di vapore
40 mmHg ( 0 °C)
Grado
purum p.a.
Saggio
≥98.0% (KT)
Forma fisica
powder
Impurezze
≤2% water
Punto di fusione
724 °C (lit.)
Anioni in tracce
sulfate (SO42-): ≤50 mg/kg
Cationi in tracce
Ca: ≤50 mg/kg
Cd: ≤50 mg/kg
Cu: ≤50 mg/kg
Fe: ≤50 mg/kg
K: ≤100 mg/kg
Na: ≤200 mg/kg
Ni: ≤1000 mg/kg
Pb: ≤50 mg/kg
Zn: ≤50 mg/kg
Stringa SMILE
Cl[Co]Cl
InChI
1S/2ClH.Co/h2*1H;/q;;+2/p-2
GVPFVAHMJGGAJG-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Acylation of various alcohols (primary and secondary).
- Acylation of various hydroxyl compounds (high selectivity).
- Regioselective ring-opening reaction of oxiranes.
- Coupling reactions of anhydrides or acid chlorides with thiols.
- Protection of aldehydes, via chemoselective dithioacetalization reaction.
- Solvent-free synthesis of β-enaminones and β-enamino esters.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Codice della classe di stoccaggio
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.