W288608
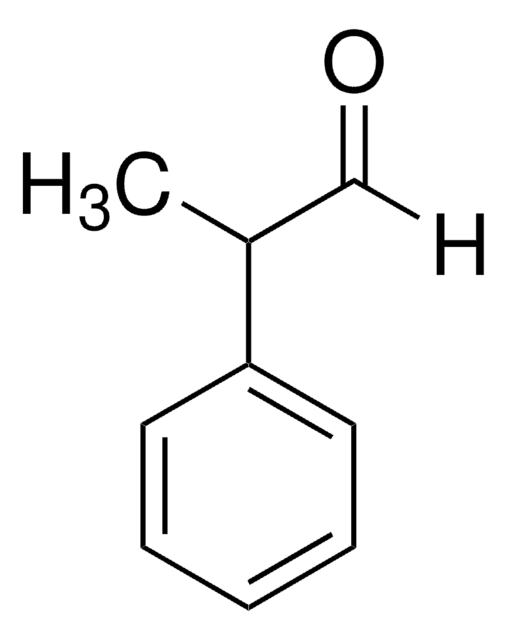
2-Phenylpropionaldehyde
≥95%, FCC, FG
Sinonimo/i:
2-Phenylpropanal, Hydratropaldehyde
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Grado
FG
Halal
Kosher
agenzia
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
Saggio
≥95%
Indice di rifrazione
n20/D 1.517 (lit.)
P. eboll.
92-94 °C/12 mmHg (lit.)
Densità
1.002 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Organolettico
fresh; green; floral
Temperatura di conservazione
2-8°C
Stringa SMILE
[H]C(=O)C(C)c1ccccc1
InChI
1S/C9H10O/c1-8(7-10)9-5-3-2-4-6-9/h2-8H,1H3
IQVAERDLDAZARL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Cytotoxicity, early safety screening, and antimicrobial potential of minor oxime constituents of essential oils and aromatic extracts.: Explores the safety and effectiveness of 2-Phenylpropionaldehyde among other compounds in essential oils, highlighting its potential antimicrobial properties and implications for food safety and preservation (Strub DJ et al., 2022).
- Spectroscopic Evidence for a Cobalt-Bound Peroxyhemiacetal Intermediate.: This study provides spectroscopic evidence of a cobalt-bound intermediate in reactions involving 2-Phenylpropionaldehyde, advancing our knowledge of chemical reaction mechanisms and catalysis (Cho J et al., 2021).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
174.2 °F
Punto d’infiammabilità (°C)
79 °C
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.