S4921
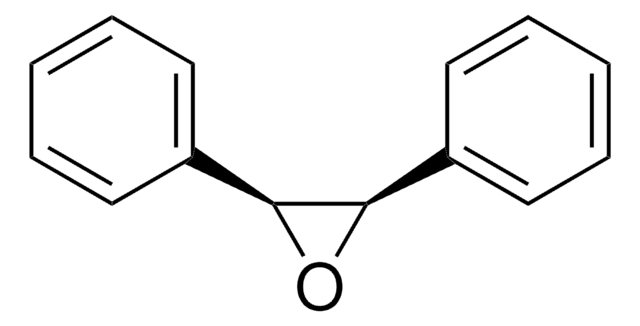
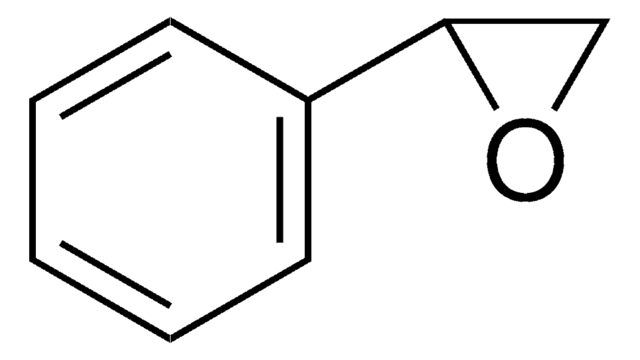
trans-Stilbene oxide
98%
Sinonimo/i:
trans-1,2-Diphenyloxirane
About This Item
Prodotti consigliati
Saggio
98%
Punto di fusione
65-67 °C (lit.)
Stringa SMILE
O1[C@@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14-/m1/s1
ARCJQKUWGAZPFX-ZIAGYGMSSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Chiral Stationary Phases for Liquid Chromatography: Trans-stilbene oxide has been utilized in the fabrication of cellulose derivative-coated spherical covalent organic frameworks, serving as chiral stationary phases for high-performance liquid chromatographic enantioseparation, demonstrating its pivotal role in advanced analytical methodologies (Yan et al., 2022).
- Method Selection for Chiral High-Performance Liquid Chromatography: Its application extends to the utilization of hysteresis phenomena for chiral high-performance liquid chromatographic method selection in polar organic mode, enhancing the efficiency and specificity of pharmaceutical compound analysis (Horváth et al., 2020).
- Adsorption Properties for Enantioseparations: The effect of chiral selector loading on the adsorption properties of fully- and superficially-porous particles is crucial for high-efficient ultrafast enantioseparations, where trans-stilbene oxide derivatives play a significant role (Felletti et al., 2018).
- Catalysis in Alkene Epoxidation: Trans-stilbene oxide is involved in innovative catalysis research, specifically in the development of carbon nitride-supported Fe(2) cluster catalysts for alkene epoxidation, showcasing its utility in sustainable chemical synthesis (Tian et al., 2018).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.