675792
(S)-(−)-2,2′,6,6′-Tetramethoxy-4,4′-bis(diphenylphosphino)-3,3′-bipyridine
97%
Sinonimo/i:
(S)-P-Phos
About This Item
Prodotti consigliati
Saggio
97%
Attività ottica
[α]20/D -98°, c = 1 in chloroform
Punto di fusione
261-265 °C
Stringa SMILE
COc1cc(P(c2ccccc2)c3ccccc3)c(c(OC)n1)-c4c(OC)nc(OC)cc4P(c5ccccc5)c6ccccc6
InChI
1S/C38H34N2O4P2/c1-41-33-25-31(45(27-17-9-5-10-18-27)28-19-11-6-12-20-28)35(37(39-33)43-3)36-32(26-34(42-2)40-38(36)44-4)46(29-21-13-7-14-22-29)30-23-15-8-16-24-30/h5-26H,1-4H3
JZOSBBLJKXSBBN-UHFFFAOYSA-N
Applicazioni
- In the asymmetic hydrogenation reactions.
- For the preparation of chiral ketone functionalized polymers by copolymerization reaction.
- To synthesize chiral alkynes by asymmetric hydroalkynylation of nonpolar alkenes or norbornadienes using iridium catalyst.
- In the selective allylic alkylation of indoles using palladium catalyst.
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
We present an article concerning P-Phos, PhanePhos and BoPhoz™ Ligands.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.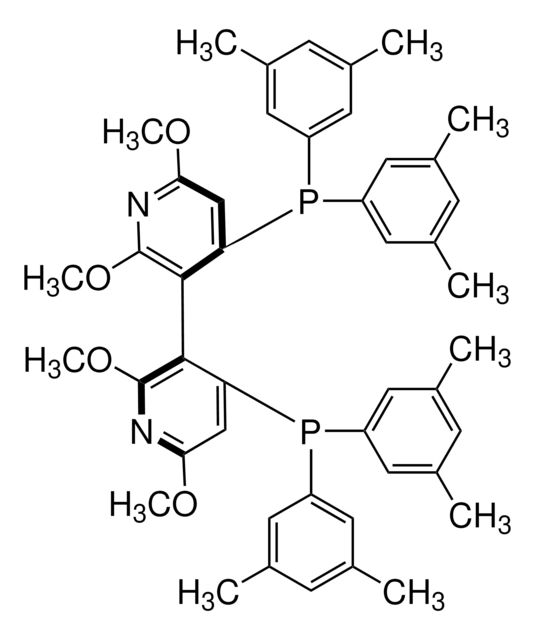
![(11bR, 11′bR)-4,4′-9,9-Dimethyl-9H-xanthene-4,5-diyl)bis-dinaphtho[2,1-d:1′, 2′-f][1,3,2]dioxaphosphepin](/deepweb/assets/sigmaaldrich/product/structures/198/331/bd88130d-f49e-4bc8-b82e-5e43b3bcea95/640/bd88130d-f49e-4bc8-b82e-5e43b3bcea95.png)


![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)