An Introduction to Antibodies: Antigens, Epitopes and Antibodies
During the first half of the 20th century, a series of scientific discoveries resolved that antibody-mediated immunity is the cornerstone of the specific immune response. Since their first use as immunolabeling research tools in the early 1970s, antibody technologies have vastly improved, and antibodies have become critical tools for most areas of life science research. The basic principle of any immunochemical technique is that a specific antibody will combine with its specific antigen to generate an exclusive antibody-antigen complex. In the following pages we will discuss the nature of this bond, and the use of this robust and specific binding as a molecular tag for research.
Antigens
The term antigen is derived from antibody generation, referring to any substance that is capable of eliciting an immune response (e.g., the production of specific antibody molecules). By definition, an antigen (Ag) is capable of combining with the specific antibodies formed by its presence.
Generally, antigens are foreign proteins or their fragments that enter host body via an infection. However, in some cases, the body’s own proteins may act as antigens and induce an autoimmune response. Bacteria and viruses contain antigens, either on their surface, or inside. These antigens can be isolated and used to develop vaccines.
Antigens are generally of high molecular weight, and commonly are proteins or polysaccharides. Polypeptides, lipids, nucleic acids, and many other materials can also function as antigens. Immune responses may also be generated against smaller substances, called haptens, if these are chemically coupled to a larger carrier protein, such as bovine serum albumin, keyhole limpet hemocyanin (KLH), or other synthetic matrices. A variety of molecules such as drugs, simple sugars, amino acids, small peptides, phospholipids, or triglycerides may function as haptens. Thus, given enough time, just about any foreign substance will be identified by the immune system and evoke specific antibody production. However, this specific immune response is highly variable and depends much in part on the size, structure, and composition of antigens. Proteins or glycoproteins are considered as the most suitable antigens due to their ability to generate a strong immune response; in other words, they are strongly immunogenic. Antigens are recognized by the host body by two distinct processes (1) by B cells and their surface antibodies (sIgM) and (2) by the T cell receptor on T cells. Although both B and T cells respond to the same antigen, they respond to different parts of the same molecule. Antibodies on the surface of B cells can recognize the tertiary structure of proteins. On the other hand, T cells require antigens that have been ingested and degraded into recognizable fragments by the antigen-presenting cells. Commonly employed antigen-presenting cells are macrophages and dendritic cells. The immune response is illustrated in Figure 1. For greater detail on the natural process of antibody production, a suitable immunology textbook should be consulted.
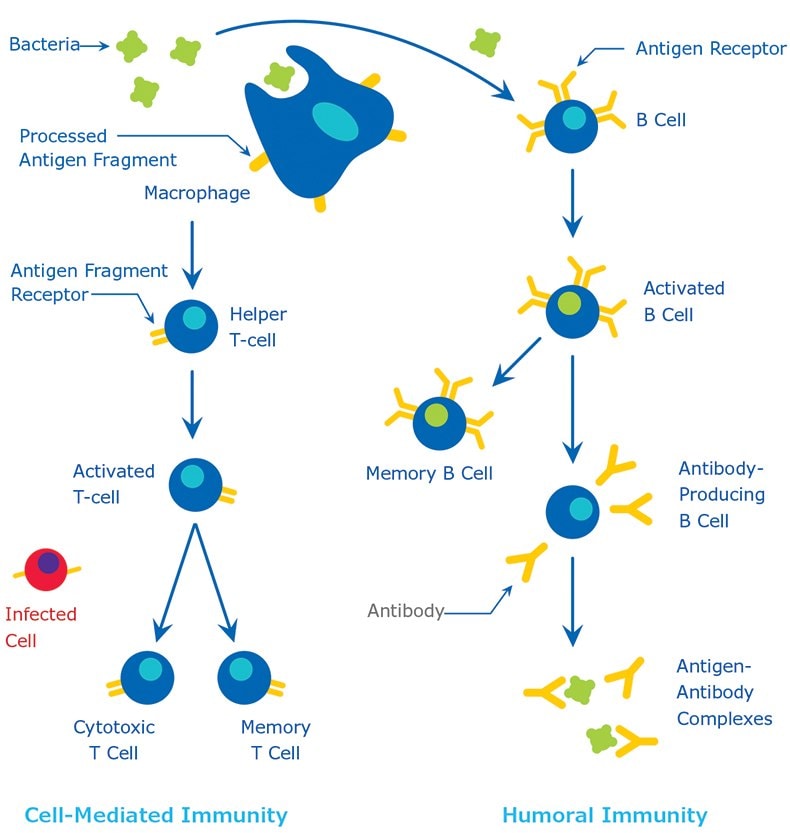
Figure 1.The Immune Response
Epitopes
The small site on an antigen to which a complementary antibody may specifically bind is called an epitope or antigenic determinant. This is usually one to six monosaccharides or five to eight amino acid residues on the surface of the antigen. Because antigen molecules exist in space, the epitope recognized by an antibody may be dependent upon the presence of a specific three dimensional antigenic conformation (e.g., a unique site formed by the interaction of two native protein loops or subunits). This is known as a conformational epitope. The epitope may also correspond to a simple linear sequence of amino acids and such epitopes are known as linear epitopes.
The range of possible binding sites on a target molecule (antigen) is enormous, with each potential binding site having its own structural properties derived from covalent bonds, ionic bonds, hydrophilic, and hydrophobic interactions. Indeed, this has important ramifications for antibody choice and performance. For efficient interaction to occur between the target antigen and the antibody, the epitope must be readily available for binding.
If the target molecule is denatured, e.g., through fixation, reduction, pH changes, or during preparation for gel electrophoresis, the epitope may be altered and this may affect its ability to interact with an antibody. For example, some antibodies are ineffective in Western blotting (WB) but are suitable for immunohistochemistry (IHC) applications, because, in the IHC procedure, a complex antigenic site might be maintained in the tissue, whereas in the WB procedure, the process of sample preparation alters the protein conformation sufficiently to destroy the antigenic site, and hence eliminates antibody binding.
In a denatured protein, only the linear epitope may be recognized. Hence, in protocols where a denatured protein is used, such as in Western blotting, an antibody that recognizes a linear epitope is preferred. Sometimes an epitope is on the interior of a folded protein. The epitope is then inaccessible to the antibody in a nondenaturing protocol, such as immunoprecipitation. A conformational epitope, by definition, is on the outside of the folded protein. An antibody that recognizes the conformational epitope is suitable for mild, nondenaturing procedures, such as immunoprecipitation or flow cytometry.
Optimally, an antibody that recognizes a linear epitope on the surface of a normally folded protein will work well in both nondenaturing and denaturing protocols. Thus, the epitope may be present in the antigen’s native, cellular environment, or it may be exposed only when denatured. In their natural form, antigens may be cytoplasmic (soluble), membrane-associated, or secreted. The number, location and size of the epitopes depend on how much of the antigen is presented during the antibody-making process.
Knowledge about the target protein, the epitope recognized by the antibody, sequence conservation, and the technique principles are valuable in making good antibody and protocol choices. Actual epitope mapping or sequence data, though useful, are not needed, however, to be confident in antibody specificity.

Figure 2.Amino acids forming a protein.
Antibodies
An antibody is defined as “an immunoglobulin capable of specific combination with the antigen that caused its production in a susceptible animal.” Antibodies are produced in response to the invasion of foreign molecules in the body. An antibody, abbreviated as Ab, is commonly referred to as an immunoglobulin or Ig. Human immunoglobulins are a group of structurally and functionally similar glycoproteins (82-96% protein and 4-18% carbohydrate) that confer humoral immunity.
Structure
Antibodies exist as one or more copies of a Y-shaped unit, composed of four polypeptide chains. Each Y contains two identical copies of a heavy chain and two identical copies of a light chain, named as such by their relative molecular weights. This Y-shaped unit is composed of the two variable, antigen-specific F(ab) arms, which are critical for actual antigen binding, and the constant Fc “tail” that binds immune cell Fc receptors and also serves as a useful “handle” for manipulating the antibody during most immunochemical procedures. The number of F(ab) regions on the antibody corresponds with its subclass (see below), and determines the valency of the antibody (loosely stated, the number of “arms” with which the antibody may bind its antigen).

Figure 3.Antibody Structure
These three regions can be cleaved into two F(ab) and one Fc fragments by the proteolytic enzyme, papain, or into just two parts: one F(ab’)2 and one Fc at the hinge region, by pepsin. Fragmenting IgG antibodies is sometimes useful because F(ab) fragments will not precipitate the antigen, and will not be bound by immune cells in live studies because of the lack of an Fc region.
Subclasses
Antibodies can be divided into five classes: IgG, IgM, IgA, IgD, and IgE, based on the number of Y units and the type of heavy chain. Heavy chains of IgG, IgM, IgA, IgD, and IgE, are known as g, µ, a, d, and e, respectively. The light chains of any antibody can be classified as either a kappa (κ) or lambda (λ) type (based on small polypeptide structural differences); however, the heavy chain determines the subclass of each antibody.
The subclasses of antibodies differ in the number of disulfide bonds and the length of the hinge region. The most commonly used antibody in immunochemical procedures is of the IgG class because this is the major immunoglobulin class released in serum.
IgA: In the blood IgA are present in low levels in monomeric form. They are most active at mucosal surfaces where they are present in dimeric form and provide the primary defense at mucosal surfaces. More IgA is produced in mucosal linings than all other types of antibody combined. Its major function is to act as a neutralizing antibody. High levels of IgA are present in saliva, tears, and breast milk. In humans two IgA subtypes are known to exist whereas in mice only one form is reported. IgA1 may account up to 85% of the total IgA in serum. Selective IgA deficiency is one of the most common immunodeficiency diseases that increases susceptibility to infections. IgA deficiencies are commonly seen in patients with autoimmune diseases and allergic disorders. IgA has a half-life of about 5 days.
IgD: It is a monomeric antibody with two epitope binding sites and is found on the surface of most B lymphocytes. Its precise function is still disputed, but is suggested to acts as an antigen receptor required for B cell activation. IgD is also reported to bind to basophils and mast cells and activate them to produce antimicrobial factors. It’s also believed to play a role in eliminating B-lymphocytes that produce self-reactive autoantibodies. IgD is also produced in a secreted form that is found in serum in small quantities and contains two heavy chains of the δ class and two light chains. IgD has a half life of about 3 days.
IgE: This group of antibodies is effective at mucosal surfaces, blood, and tissues. It is present as monomer consisting of two heavy chains (ε chain) and two light chains. The ε chain contains 4 Ig-like constant domains. In serum, it is present in low concentrations contributing to only about 0.002% of total serum antibodies. Most IgE is tightly bound to its receptors on mast cells and basophils via the Fc region. It plays a crucial role in hypersensitivity reactions and its production is strictly controlled by cytokines. IgE has a half-life of about 2 days.
IgG: This is the most abundant class of antibodies in the blood, comprising up to 80% of the total serum antibodies. It is present in monomeric form. Four subclasses of IgG have been described depending on their abundance (IgG1>IgG2>IgG3>IgG4) and the subclass produced is dependent on the type of cytokine present.
IgG1 and IgG3 exhibit high affinity for Fc receptors on phagocytes, while IgG2 exhibits very low affinity and IgG4 has moderate affinity for Fc receptors IgGs are capable of exiting the circulatory system and enter tissues. IgG1, IgG3, and IgG4 can cross placental barrier to provide protection for newborns. IgGs are efficient at activating the complement system, and are very effective for opsonization using Fc receptors on phagocytes. Through its Fc region IgG can also bind to natural killer cells and participate in antibody-dependent cytotoxicity. IgG has a half-life ranging from 7 to 23 days, depending on its subclass.
IgM: This class of immunoglobulin is first to be produced in response to infection and is found either on membranes of B cells or as a 5-subunit macromolecule secreted by plasma cells. It is also the first immunoglobulin class to be synthesized by the neonates. The surface IgM differs from the secreted form in its Fc region. Surface IgM binds directly as an integral membrane protein and not to the IgM Fc receptor. Secreted IgM is a pentameric molecule where multiple immunoglobulins are covalently linked with disulfide bonds. This structure provides multiple binding sites. Each monomer consists of two light chains (either κ or λ) and two heavy chains. Because of its pentameric nature IgM is particularly suited for activating complement and causing agglutination. IgM has a half-life of about 5 days.

Figure 4.Heavy chain-only antibodies.
A heavy-chain shark antibody (IgNAR) and a heavy-chain camelid antibody (hclgG) in comparison to a common antibody (IgG). Heavy chains are shown in a darker shade, light chains in a lighter shade.
Continue learning about Antibodies with Antibody- Antigen Interaction
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?