MALDI-TOF Characterization of Functionalized Polymers
Molly E. Payne, Scott M. Grayson
Department of Chemistry, Tulane University, New Orleans, Louisiana
With an increased demand for high performance and specialized polymeric materials, tools for selective end group functionalization and modification have been a focus of synthetic development. Due to their tunable physical and chemical properties, polymers are an essential component for a variety of materials1, bioconjugations2, macromonomers3, and biomedical materials4.
Due to their complexity and functional moieties, many target complexes (e.g., biologically-active materials) are incompatible with polymerization conditions. To mitigate these issues, a wide range of synthetic methodologies have been developed for efficient post-polymerization conjugation. For example, end groups are frequently added to polymers for use in high-efficacy coupling reactions such as azide-alkyne coupling5 and thiol-ene coupling6.
MALDI-TOF MS for Polymer Characterization
A critical limitation to developments in polymer end-group functionalization lies in the difficulty in confirming end-group purity before and after modification. Modern characterization techniques, especially matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS), have proved invaluable in confirming end group fidelity. MALDI-TOF is a soft ionization technique that enables resolution of individual n-mers of polymers in a mass spectrum distribution. This resolution enables the elucidation of not only mass distribution and repeat unit mass, but also the identity and fidelity of end groups.
Molecular Weight Characterization
For relatively low dispersity polymers (Đ≤1.3), MALDI-TOF MS can be used to accurately determine mass distribution data. The number average molecular weight (Mn) is the mass average with respect to the number of moles of each mass fraction and can be calculated using the following formula:
where Ni = number of molecules of a specific molecular weight and Mi = the specific molecular weight of those molecules
The weight average molecular weight (Mw) is the mass average with respect to the weight of each mass fraction and can be calculated using the formula:
where Ni = number of molecules of a specific molecular weight and Mi = the specific molecular weight of those molecules
The breadth of the molecular weight distribution, or dispersity (Đ), can be quantified using the ratio of Mw/Mn.
End group characterization
In addition to its ability to quantify polymer molecular weight distributions, MALDI-TOF MS can also be used to resolve homopolymer end groups. This can be accomplished by rearranging the following formula for the observed mass of an n-mer in the mass spectrum (Mn-mer):
Mn-mer = n(MRU) + MEG1 + MEG2 + Mion where n = the degree of polymerization,
MEG1 = mass of the α-end group,
MEG2 = mass of the ω-end group,
MRU = the mass of the repeat unit of the polymer
and Mion = mass of the ion that complexes with the polymer.
MALDI-TOF characterization of commercially-available materials
A wide variety of ready-to-use, functionalized polymers are commercially available for biomedical applications, such as PEGylation, hydrogel synthesis, targeted drug delivery, and bioconjugation. We have used MALDI-TOF to verify the dispersity and functionality of a selection of highly-defined, functionalized materials currently available. All of the MALDI-TOF spectra provided below show the full spectrum with an inset of an individual n-mer. Our results verify both the narrow dispersity of these materials as well as identity and degree of functionalization of these select materials.
Product 689696: Poly(ethylene glycol) bis-azide (Mn=2000)
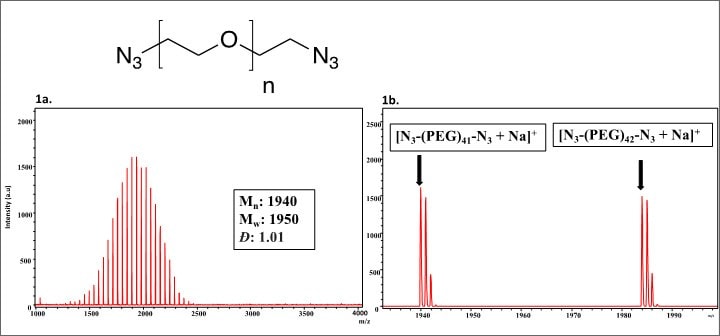
Figure 1.Visual observation of the spectra confirms that the polymer has a narrow, monomodal distribution. The molecular weight distributions for this polymer were calculated to be Mn: 1940 and Mw: 1950, which leads to a narrow distribution with Đ = 1.01. The peaks within the spectrum correspond to a single distribution with each peak separated by 44.026 mass units, suggesting that only one set of end groups exists within this sample. The end groups can be verified by looking at individual n-mers, such as the 41-mer [Figure 1b]. The theoretical mass value of the 42-mer with two azide end groups is 1984.13978 which was calculated by multiplying the repeat unit mass (44.02621) by the number of repeat units (42), adding the mass of the azido end group (42.00922), adding the mass of the azidoethyl end group (70.04052), and finally adding the mass of the sodium cation (22.98922). The observed value for the 42-mer is 1983.95 which is 0.19 Da different from the theoretical value.
Product 747386: Poly(L-lactide), thiol terminated (Mn=2,500)
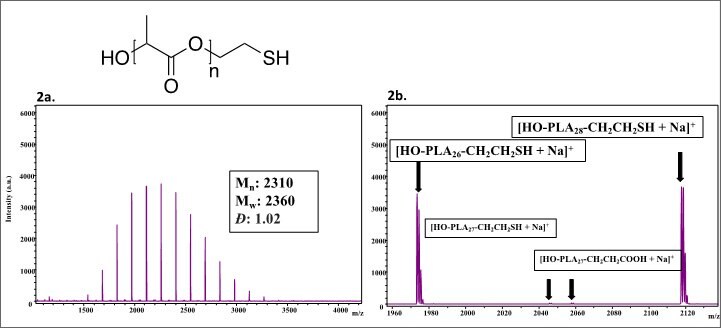
Figure 2.Visual observation of the spectra confirms that the polymer has a narrow, monomodal distribution. The molecular weight distributions for this polymer were calculated to be Mn: 2300, Mw: 2350, and Đ =1.02. The major peaks within the spectra correspond to a single distribution with each peak separated by 72.021 mass units, suggesting that only one set of end groups exists within this sample. The end groups were verified by looking at the individual 26-mer [Figure 2b]. The theoretical mass value of the 26-mer with -hydroxy and ω-thiol end groups is 1973.55254 which is 0.07 Da different from that of the observed mass of 1973.62 Da. Very minor peaks exist in between every major peak in this material. Looking specifically at the minor peaks observed in the inset, the peak at 2045.74 corresponds to the 27-mer of the polymer and the peak at 2057.73 corresponds to a carboxylic acid terminated polymer (instead of the thiol functionality). These minor impurities are likely due to either water initiation in the ring opening polymerization of the lactide monomer or transesterification.
MALDI-TOF characterization of polymers after conjugation reactions
While many types of reactions are used to facilitate polymer conjugation, click reactions are widely used due to their ease, efficiency, and broad functional group compatibility. Popularized by Sharpless et al.7, copper-catalyzed azide-alkyne cycloaddition (CuAAC)5 conjugates an alkyne reagent to an azide reagent via the formation of a 1,2,3-triazole ring. The Bertozzi group developed strain-promoted alkyne-azide cycloaddition (SPAAC)8 between an azide and cyclooctene reagent, as a copper-free alternative for use with live cells and in vivo. Thiol-ene click reactions6 between an alkene and thiol reagent to form an alkyl sulfide are also useful metal-free alternatives due to their compatibility for a range of applications, especially biological applications. Additionally, as amines are ubiquitous in nature and biology, amidation reactions9 between carboxylic acids and amines are particularly important in the synthesis of peptides, proteins, and their conjugates.
While click reactions feature high conversion, confirmation of reaction completion can be difficult for polymer conjugation reactions. Due to the low relative concentration of the newly formed linkage in comparison to the polymer repeat units, traditional characterization methods (e.g., 1H NMR) are typically not sensitive enough for verification. In contrast, MALDI-TOF can be used to resolve the end groups and confirm reaction completion. The examples shown below feature two different types of click reactions and validate the desired compound was formed. The MALDI-TOF spectra provided for each reaction show the spectrum of the starting material above and the final product below.
Modification of poly(ethylene glycol) bis-azide (Product 689696) with 1-ethynyl-4-fluorobenzene via CuAAC
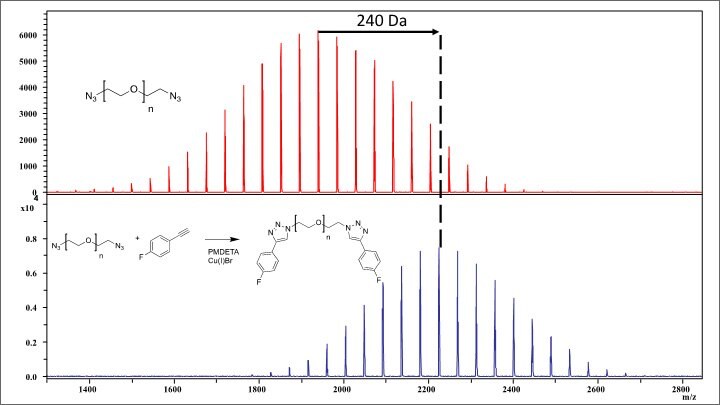
Figure 3.Shows MALDI-TOF spectra of polyoxyethylene bis(azide) (Mn=2000) (red) modified with 1-ethynyl-4-fluorobenzene via an azide-alkyne cycloaddition (blue). Visual observation of the spectra confirms that a modification has occurred, but this can also be confirmed based upon the mass difference between the selected n-mers. As shown by the black arrow, there is an observed shift of 240.21 Da between the starting material and the product. This corresponds to the difference in the 42-mer of the starting material (1983.95) compared to the 42-mer of the product (2224.16) and is in close agreement with the theoretical exact mass difference of 240.08.
Modification of thiol-terminated poly(L-lactide)(Product 747386) with 2,5-pyrroledione via thiol-ene click reaction
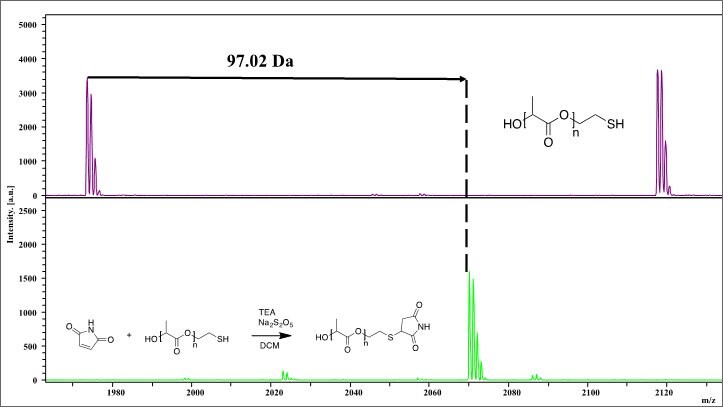
Figure 4.Shows MALDI-TOF spectra of thiol-terminated poly(L-lactide) (purple) modified with 2,5-pyrroledione (green) via a thiol-ene coupling. Visual observation of the entire spectra confirms that the starting material underwent some type of modification, but completion of the thiol-ene reaction can be confirmed by examining the shift of the individual n-mers. Indicated by the black arrow, a shift of 97.02 Da from 26-mer of the starting material (1973.55) to the 26-mer product (2214.56) is observed, which corresponds to the mass shift that would be expected post thiol-ene reaction.
Conclusion
Commercially available with a variety of molecular weights, compositions, and end group functionalities, polymers have become a critical component for many applications. With the proper end group, functionalized polymers can provide a quick and efficient route for the synthesis of a wide variety of polymer conjugates. As the complexity of these materials increases to meet the needs of researchers, MALDI-TOF MS is a valuable tool for confirming end group identity and transformations, verifying successful and complete conjugation.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?