NanoFabTx™: A ready-to-use Microfluidic Platform for mRNA Vaccine Formulation
Ben Knappett<sup>1</sup>, Richard Gray<sup>1</sup>, Zhen Ye<sup>2</sup>, Rajiv Kumar<sup>2</sup>, Elizabeth Aisenbrey<sup>2</sup>, Nicolynn Davis<sup>2</sup>
1Dolomite Microfluidics, 2Merck
The COVID-19 pandemic greatly accelerated vaccine research, with mRNA at the forefront of those efforts. In order for mRNA vaccines to be effective, a carrier that can deliver the vaccine to its target location and protect the mRNA from rapid degradation in the body is essential. This requirement led to the development of multiple lipid-based carriers, also known as lipid nanoparticles (LNPs) or liposomes. While conventional production of lipid-based carriers involves lengthy trial-and-error optimization, alternative approaches using microfluidics can streamline early-stage vaccine development. Our NanoFabTx™ reagent and microfluidic device kits provide a ready-to-use platform to screen multiple formulations, and ultimately synthesize mRNA or drug-encapsulated liposomes for preclinical assessments.
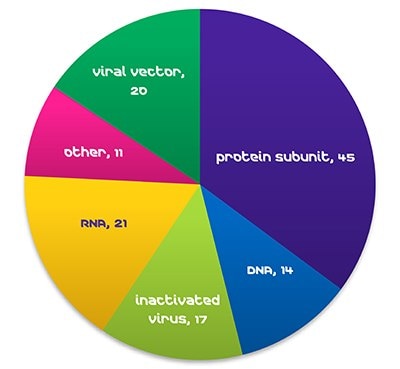
Figure 1. COVID-19 Vaccines in Clinical Development, WHO, October 29, 2021.Global demand for vaccines is expanding rapidly, with >25% annual growth in doses during relatively normal times (WHO, 2019). The advent of new viruses, such as Ebola, MERS, SARS, and the current COVID-19 pandemic, is leading to a dramatic increase in demand, and strong pressure to shorten development. As of late October 2021, RNA vaccines make up 17% of COVID-19 vaccines in clinical development (WHO).
What are mRNA vaccines?
Unlike traditional vaccines which often use a weakened or killed form of a pathogen, chemically synthesized mRNA (or messenger RNA) vaccines carry only enough information to teach our cells to make a small part of a pathogen. Once the pathogen fragment is produced by our cells, our immune system recognizes that it does not belong and triggers an immune response which protects us from future infection. It is not possible for our cells to make the whole pathogen from an mRNA vaccine, increasing safety.
Additionally, mRNA vaccines have been found to generate a reliable immune response and are well-tolerated by healthy individuals with few side effects. Because mRNA vaccines can be created in a laboratory and do not require cell culture, production is more rapid and standardized, which can potentially improve responsiveness to emerging outbreaks.
RNA is a fragile molecule and would be broken down in the body by natural enzymatic processes in its free state. Delivering mRNA successfully into cells is therefore a key challenge in mRNA vaccine development. Encapsulating mRNA in lipid carriers (liposomes and LNPs) is an ideal way to ensure that a mRNA vaccine can successfully enter cells and be delivered into the cytoplasm (Figure 2).
mRNA vaccine delivery via lipid carriers
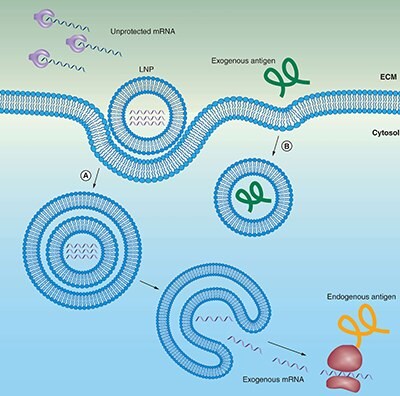
Figure 2.Lipid carriers (liposomes) protect mRNA from degradation and facilitate endocytosis and endosomal escape.
(A) mRNA can be encapsulated in liposomes for protection from enzymatic degradation. A positively charged liposomes favors localization of mRNA at the negatively charged cell membrane, including subsequent endocytosis into the cytosol. To reach the cytoplasm to get transcribed, the mRNA must escape both the endosome and lipid carrier.
(B) Extracellular protein-based vaccines are endocytosed in a similar manner, but do not need to escape from the endosome to be presented on MHCII.
Features of lipid carriers:
- Lipid nanocarriers closely resemble the structure of cell membranes
- They can be used to deliver both hydrophobic and hydrophilic drugs
- Their natural composition and nano-size enable them to be effective carriers, reaching internal tissues and cells to deposit active pharmaceutical ingredients, nucleic acids, proteins, DNA or RNA strains
- Through surface decoration with ligands and/or co-delivery with adjuvants, lipid carriers can target desired cell types
Lipid selection is a key factor for mRNA delivery and requires a series of steps to optimize specific lipid composition, size, stability, charge, surface functionalization, as well as mRNA encapsulation and release efficiency. Moderna and Pfizer mRNA lipid-based vaccines demonstrate the importance of this technology in the epidemiological battle against COVID-19 disease.
Microfluidics for streamlined and high-throughput lipid optimization
Conventionally, lipid carriers have been produced by batch methods (Figure 3B), by slowly adding lipid solution into an agitated aqueous phase. This approach gives poor size control of the resulting LNP, low encapsulation efficiency, and requires revalidation each time the process is scaled from laboratory to pilot plant to full production. Microfluidic methods (Figure 3A) can help with each of these areas.

Figure 3. Schematic representation of the process of liposome formation.
(A) microfluidic liposome self-assembly
(B) standard batch ethanol injection procedure
Advantages of microfluidics for mRNA nanoparticle encapsulation:
- Reduce lead times
- Increase efficiency and quality
- Decrease development costs
- High-throughput capabilities
- Can be automated and readily scaled for production
How does it work?
Microfluidic synthesis methods directly mix the lipid–organic phase with an aqueous solution of mRNA in a small mixer chip, where fast and reproducible lipid carrier formation and RNA encapsulation take place. Recently, automated microfluidic solutions have been developed. For example, the Dolomite Automated Nanoparticle System (ANPS) increases productivity and reduces development lead time by automatically generating multiple samples under different process conditions. Once optimized, the system can run continuously to produce large amounts of material for in vitro and in vivo trials.
Introduction to the microfluidic NanoFabTx™ kits for lipid carrier optimization
NanoFabTx™ nanoformulation device and reagent kits are well suited for mRNA vaccine development because they address the two main challenges in development: (1) lipid formulation selection and optimization and (2) scalable assembly methods.
The NanoFabTx™ device and reagent kits offer:
- A streamlined strategy for screening and selecting desired formulations without the need for lengthy trial-and-error optimization
- A selection of various lipid formulations that have been studied and selected for drug/RNA delivery
- A microfluidic device kit to create mRNA-encapsulated liposomes with narrow size dispersity and high batch-to-batch consistency
- Comprehensive protocols, pre-assembled microfluidic chip manifolds, and required accessories compatible with the Dolomite microfluidics system, syringe pumps or traditional nanoprecipitation methods
The resulting drug or nucleic acid-loaded lipid or polymeric carriers are biocompatible and biodegradable and can be further modified to target specific tissues or to ensure slow and sustained drug release.
Controlling size and composition using the NanoFabTx™ microfluidic nano device kit
The NanoFabTx™ device kit can be used to test various formulation parameters without lengthy trial and error optimization. Liposome size can be fine-tuned by optimizing parameters such as the flow rate ratio (FRR) (Figure 4A). Using the NanoFabTx™ microfluidic nano device kit, liposomes of consistent size can be synthesized regardless of formulation or lipid concentration (Figure 4B). This rapid lipid screening is imperative to early-stage vaccine development and often unachievable with conventional lipid film hydration/extrusion methods.
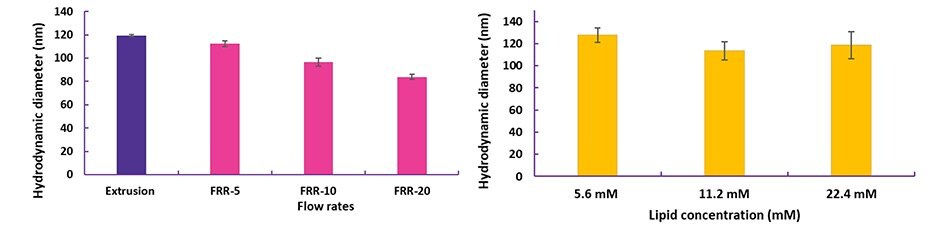
Figure 4. Optimization of liposomes prepared with NanoFabTx™ microfluidic - nano device kit
(A) PEGylated liposomes encapsulating a small molecule were synthesized at different flow rate ratios (FRR, Organic:aqueous).
(B) Cationic liposomes encapsulating siRNA were synthesized at different total lipid concentrations. Liposome sizes were measured using dynamic light scattering (DLS).
Intracellular delivery of siRNA-loaded liposomes prepared using NanoFabTx™ reagent and device kits
The delivery and uptake of siRNA-loaded liposomes developed using the NanoFabTx™ reagent and device kits have been assessed in multiple applications. First, the uptake of cationic liposomes loaded with fluorescently labeled FAMsiRNA was demonstrated in A549 lung cancer cells in vitro (Figure 5). No uptake of the naked FAMsiRNA was observed. In comparison, over 90% of cells treated with the liposome loaded FAMsiRNA (Lipo- FAMsiRNA) show a strong fluorescent signal indicating a robust uptake of FAMsiRNA when complexed with cationic liposomes.
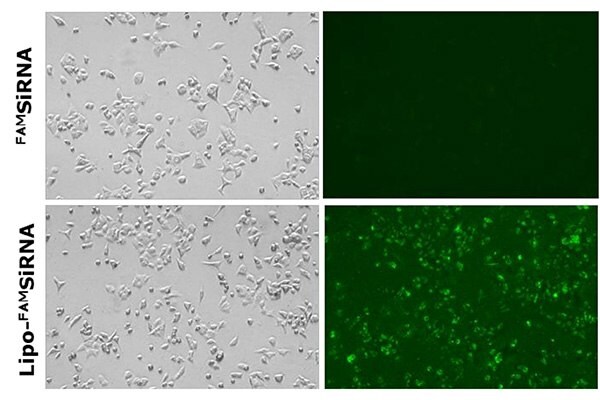
Figure 5.Delivery of naked FAMSiRNA (upper panel) and FAMSiRNA complexed with cationic liposome formulation (lower panel) in lung cancer cell line A549. All images were acquired using a fluorescence microscope.
The delivery of siRNA and its ability to silence GFP-labeled HeLa cells in vitro was also assessed. Liposomes of varying N/P ratios (Nitrogen (N) of DOTAP/Phosphate (P) of siRNA) were prepared using the NanoFabTx™ reagent and device kits and screened for optimal transfection efficiency. N/P ratios of 7.5 and 5 resulted in similar transfection efficiencies and reduced GFP expression by approximately 40% compared to the untreated control cells. Furthermore, to optimize GFP-siRNA knock-down efficiency, three different sequences of siRNA were screened out. Two sequences (siGFP-126 and siGFP-240) resulted in 40-50% silencing (reduced GFP expression) in GFP-HeLa cells (Figure 6C&D). These results suggest liposomes prepared by the NanoFabTx™ platform not only delivered the siRNA into GFP-HeLa cells, but also effectively silenced the target gene. Additionally, a head-to-head comparison between liposome preparation methods (microfluidics vs. extrusion) demonstrated similar transfection efficiencies, though microfluidics was found to be easier than the labor-intensive extrusion method.
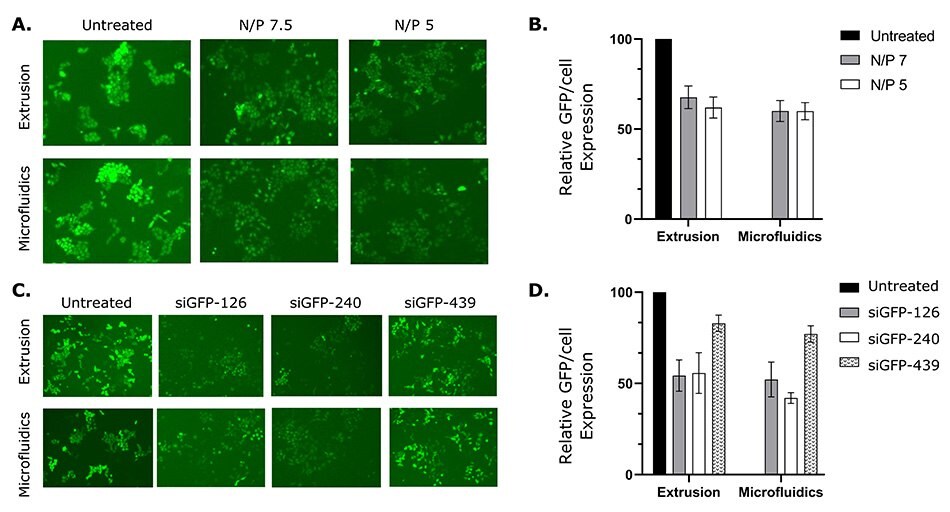
Figure 6.siRNA-loaded liposomes prepared with NanoFabTx™ reagent and device kits silence GFP expression in GFP-HeLa cells.
(A) Representative fluorescent images and
(B) relative GFP/cell expression (GFP/cell expression relative to untreated control) of GFP-HeLa cells treated with liposome-siRNA at varying N/P ratios.
(C) Representative fluorescent images and
(D) relative GFP/cell expression of GFP-HeLa cells treated with liposome-siRNA complexed with different siRNA sequences.
NanoFabTx™ applications in vaccine development
These demonstrate the capabilities of the NanoFabTX™ reagent and device kits in synthesizing a variety of liposomes for the delivery of RNA and other therapeutics. The flexibility of the NanoFabTX™platform enables optimization by screening parameters such as formulation and flow rate ratio until a desired liposome size, drug encapsulation, and targeted delivery is achieved. MilliporeSigma and Dolomite have partnered together in developing microfluidic solutions to liposome synthesis with our preassembled NanoFabTX™device kits and optimized protocols. These kits allow for rapid synthesis of well-defined particles, can be adapted for therapeutic drugs like mRNA and vaccine development, and can be scaled up using Dolomite’s Automated Nanoparticle System (ANPS) for preclinical assessment. The new ANPS, in addition to NanoFabTX™ kits, allows users to automate experiments to make small samples of liposomes, lipid nanoparticles, and other types of organic nanoparticles, to address the following key challenges in vaccinology:
- Directed evolution of bacteria strains for plasmids
- Directed evolution of optimal strain of yeast/ bacteria for vaccine production
- Antibody screening
- Development and production of adjuvant formulations
- Development and production of vaccine delivery particles
NanoFabTx™ Reagent Kits
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?