Trialkylphosphine for Morita-Baylis Hillman Reactions
The use of amines and phosphines in nucleophilic catalysis is well precedented; however, arguably one of the severe limitations with respect to exploiting the more nucleophilic, yet less basic, phosphine in this regard is its air sensitivity. This is especially true for the most nucleophilic of this class, the trialkylphosphines. While utilization of these difficult-to-handle phosphines as their trifluoroborate salts has provided an excellent solution to handling limitations, there are circumstances where the use of base is not necessary or possible. He and co-workers have demonstrated the use of the known cage-like, air-stable trialkylphosphine, 1,3,5-triaza-7-phosphaadamantane (PTA), in organocatalysis, and specifically in the Morita-Baylis-Hillman (MBH) reaction (Table 1). Traditionally, MBH reactions are conducted using DABCO®, but slow reaction rates often hamper its utility. The authors’ use of PTA has addressed some of these difficulties, utilizing mild and environmentally friendly conditions, to provide the desired products in good to excellent yields. In addition, the use of PTA is also proving successful in historically challenging cases, such as MBH reactions using acrylate as the electrophile.

Table 1.
The authors also presented reasonable evidence implicating the phosphorus-bound Michael adduct 1 by preparation of species 3 via reaction of PTA with ethyl acrylate in THF-H2O (Scheme 1). Not only does formation of adduct 3 substantiate organocatalysis through the Michael adduct, but it also proves that the tertiary alkyl phosphine is behaving as the organocatalyst and not the nitrogen.1
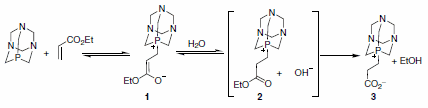
Scheme 1.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?