Neuronal Mesenchymal Stem Cells
Human mesenchymal stem cells (MSCs) represent a promising tool in regenerative medicine because of their ability to self-renew and differentiate into a variety of specialized cells including adipocytes, osteocytes, chondrocytes and myocytes. These multipotent stem cells can be easily isolated from various tissues including bone marrow, adipose tissue, umbilical cord and dental pulp. Under appropriate culture conditions, MSCs are able to transdifferentiate into neuron-like cells which opens up the possibility of using MSCs to replace damaged neurons in neural degenerative disorders or traumatic brain injuries. Differentiation of mesenchymal stem cells into cells of neuronal lineage is accompanied by striking changes in cell morphology with the formation of neural dendrites and axons. The neuronal induction process can be monitored by observing these morphological changes. The cresyl violet histochemical staining technique, which stains neuronal Nissl bodies, can also be used for the detection of neuronal cells. These characteristic granular structures are composed of RNA-rich rough endoplasmic reticulum (rER) and are unique to the somata of neurons. PromoCell’s Mesenchymal Stem Cell Neurogenic Differentiation Media (C-28015) was developed for the directed differentiation of mesenchymal stem cells (MSC) from bone marrow, the umbilical cord matrix (Wharton´s Jelly) and adipose tissue into neurogenic lineages.
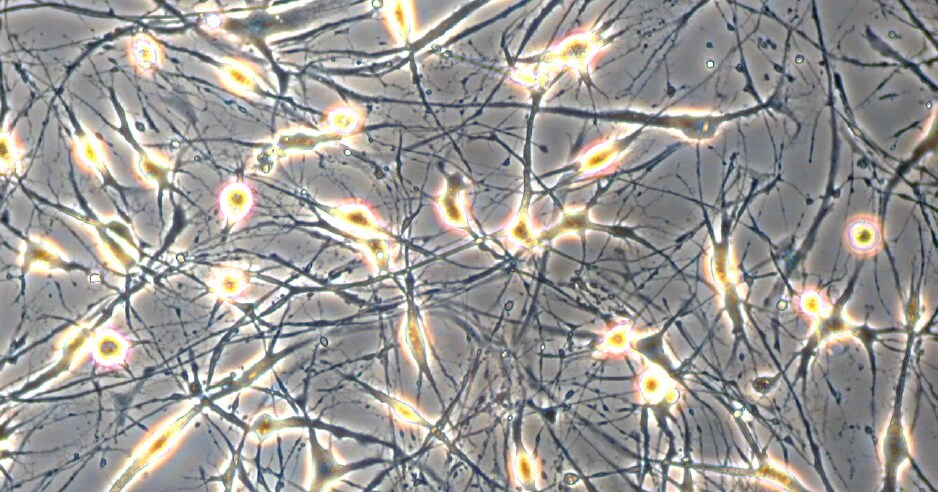
Figure 1. Neural differentiation of human MSCs. Neuron-like cells generated from human bone marrow derived mesenchymal stem cells in PromoCell’s MSC Neurogenic Differentiation Medium (C-28015). Note the formation of axon- and dendrite-like cellular structures.
Neuronal Differentiation Protocol
- Coat the culture vessel. Coat a 6-well tissue culture plate with 10 μg/mL human fibronectin or bovine fibronectin according to the instruction manual.
- Seed the Mesenchymal Stem Cells. Plate 4 x 103 cells/cm2 on the fibronectin-coated plate using MSC Growth Medium 2 (C-28009). Work in duplicate.
- Let the Mesenchymal Stem Cells grow. Culture the cells to 60-80% confluency. Change the medium every 48 hours.
- Induce the Mesenchymal Stem Cells. Induce one of the duplicate samples with MSC Neurogenic Differentiation Medium (C-28015). Use MSC Growth Medium 2 (C-28009) for the remaining well as a negative control.
- Differentiation of the Mesenchymal Stem Cells. Incubate for at least 3 days. Change the medium every 48 hours. Note: Significant morphological changes in the cells can be observed as early as 1 day after induction (Fig.1).
- Harvest and characterize the cells. The MSC-derived neuronal cells are now ready to be used in your experiments. If required, the neuronal cells can be characterized further by proceeding with the following protocol.
Detection of Neuronal Markers
Cresyl Violet/Nissl Body Staining
- Prepare reagents and buffers. Use Saccomanno Fixation Solution. Prepare the Nissl staining solution (0.5% cresyl violet) as follows: make 0.6 mL of glacial acetic acid (A6283) up to 100 mL with distilled water. Add 0.5 g of cresyl violet acetate (C5042) and stir for 20 min. Pass through a Stericup 0.22 μM filter. Store in the dark in a tightly closed container at room temperature and use within 6 months.
- Wash the cells. Remove the cells from the incubator, aspirate the medium and gently wash the cell monolayer twice with phosphate-buffered saline (PBS) w/o Ca++/Mg++ (D8537).
- Fix the cells. Aspirate the PBS (D8537) and fix the cells with Saccomanno Fixation Solution for at least 30 min at room temperature. Use enough fixative to cover the cell monolayer completely.
- Wash the cells. Aspirate the fixation solution and gently wash the cell monolayer twice with PBS (D8537).
- Stain the cells. Immediately before use, pass the required amount of Nissl staining solution through a 0.22 μM Millex PES filter. Remove the PBS from the cells and add the staining solution. Use enough staining solution to cover the cell monolayer completely. Incubate at room temperature for 30 min.
- Wash the cells. Aspirate the staining solution. Gently wash the cell monolayer three times with PBS (D8537).
- Detect results. Cover the stained cells with PBS (D8537) and evaluate the samples promptly as the dye may bleed upon prolonged storage. Use a microscope at low magnification (40-50x) in bright field mode. Results: nuclei stain a light blue/violet color, Nissl bodies appear dark black-violet with the background remaining almost colorless (Fig.2).
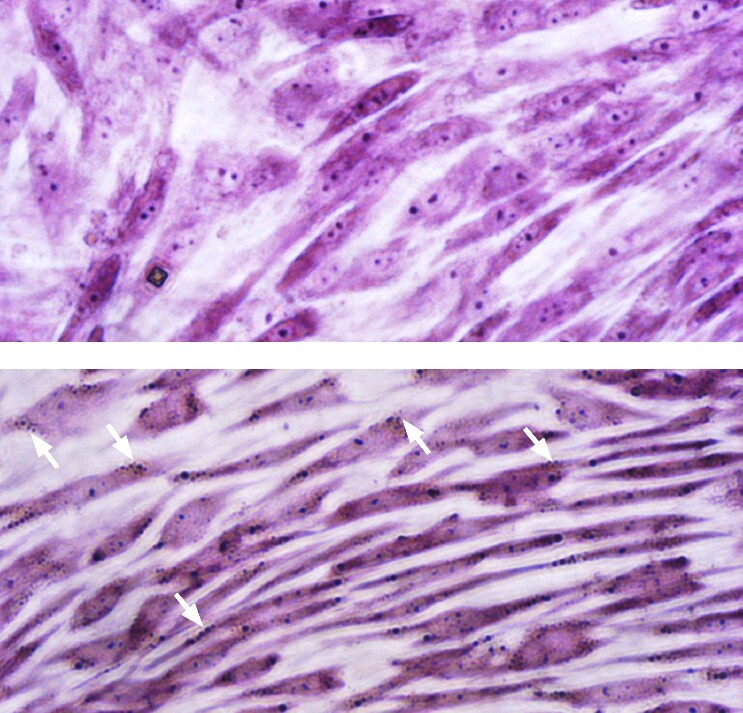
Figure 2. Nissl body staining of hMSC-BM derived neuron-like cells.Cells were cultured for 3 days in PromoCell MSC Growth Medium 2 for the negative control (top panel) or MSC Neurogenic Differentiation Medium for the differentiation sample (bottom panel). In contrast to the negative control, the neuronal cells differentiated from MSC show extensive somata-associated accumulations of Nissl bodies stained dark black-violet (white arrows).
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?