SAE0097
D-2-Hydroxyglutarate Dehydrogenase (D2HGDH) from Acidaminococcus fermentans
recombinant, expressed in E. coli, aqueous solution
Synonyme(s) :
D2HGDH, HGDH, L-2-hydroxyglutarate dehydrogenase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro de classification (Commission des enzymes):
1.1. 99.2
Code UNSPSC :
12352202
Nomenclature NACRES :
NA.54
Produits recommandés
Produit recombinant
expressed in E. coli
Pureté
≥95% (SDS-PAGE)
Forme
aqueous solution
Activité spécifique
≥1000 units/mg protein
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Température de stockage
−20°C
Description générale
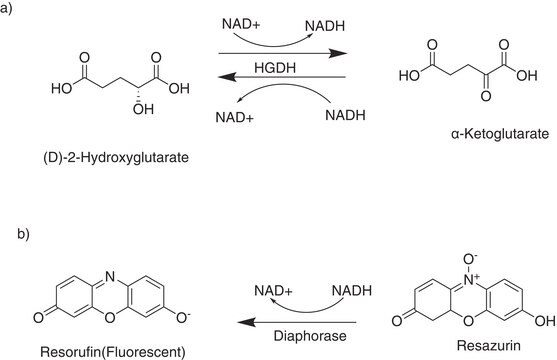
D-2-Hydroxyglutarate Dehydrogenase (D2HGDH) is a member of the D-2-hydroxyacid NAD+ dependent dehydrogenase family of proteins. D2HGDH catalyzes the conversion of α-ketoglutarate (α--KG) to D-2-hydroxyglutarate (D2HG), coupled to the oxidation of NADH to NAD+ .
The crystal structure of D2HGDH from Acidaminococcus fermentans has been reported. D2HGDH from Acidaminococcus fermentans has been used in several enzymatic assays, such as:
The crystal structure of D2HGDH from Acidaminococcus fermentans has been reported. D2HGDH from Acidaminococcus fermentans has been used in several enzymatic assays, such as:
- A continuous spectrophotometric assay to measure the activity of aminotransferases, based on the transamination of a keto compound and L-glutamate, which yields a corresponding amino compound and 2-oxoglutarate.
- Determination of D2HG levels in biological fluids such as serum, urine, cell culture supernatants, and cell or tissue lysates.
- A coupled assay system to measure branched-chain amino acid aminotransferase activity.
Définition de l'unité
One unit of enzyme oxidizes 1 μmole of NADH to NAD+ coupled to the reduction of α-ketoglutarate to (D)-2-hydroxyglutarate per minute at 37°C at pH 8.0.
Notes préparatoires
This recombinant D2HGDH product is supplied as an aqueous solution in 20 mM Trizma® buffer, pH 7.5, with 150 mM NaCl, and 10% glycerol.
Informations légales
T3P is a registered trademark of Archimica GmbH
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Berta M Martins et al.
The FEBS journal, 272(1), 269-281 (2005-01-07)
NAD(+)-dependent (R)-2-hydroxyglutarate dehydrogenase (HGDH) catalyses the reduction of 2-oxoglutarate to (R)-2-hydroxyglutarate and belongs to the d-2-hydroxyacid NAD(+)-dependent dehydrogenase (d-2-hydroxyacid dehydrogenase) protein family. Its crystal structure was determined by phase combination to 1.98 A resolution. Structure-function relationships obtained by the comparison
Xuejing Yu et al.
Analytical biochemistry, 431(2), 127-131 (2012-09-25)
A continuous general spectrophotometric assay for measuring the activity of aminotransferases has been developed. It is based on the transamination of a keto compound (amino acceptor) and l-glutamate (amino donor), yielding the corresponding amino compound and 2-oxoglutarate. The rate of
Jörg Balss et al.
Acta neuropathologica, 124(6), 883-891 (2012-11-03)
Levels of (D)-2-hydroxyglutarate [D2HG, (R)-2-hydroxyglutarate] are increased in some metabolic diseases and in neoplasms with mutations in the isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) genes. Determination of D2HG is of relevance to diagnosis and monitoring of disease.
Xuejing Yu et al.
The FEBS journal, 281(1), 391-400 (2013-11-12)
Branched-chain amino acid aminotransferase (BCAT) plays a key role in the biosynthesis of hydrophobic amino acids (such as leucine, isoleucine and valine), and its substrate spectrum has not been fully explored or exploited owing to the inescapable restrictions of previous
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique