74011
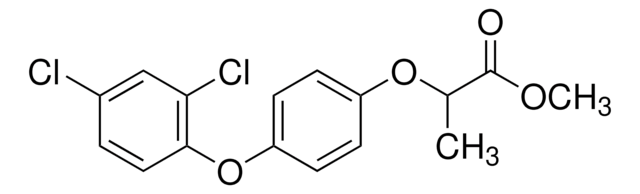
(±)-Diclofop
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonyme(s) :
(RS)-2-[4-(2,4-Dichlorophenoxy)phenoxy]propionic acid
About This Item
Produits recommandés
Qualité
certified reference material
TraceCERT®
Niveau de qualité
Gamme de produits
TraceCERT®
Durée de conservation
limited shelf life, expiry date on the label
Fabricant/nom de marque
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CC(Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1)C(O)=O
InChI
1S/C15H12Cl2O4/c1-9(15(18)19)20-11-3-5-12(6-4-11)21-14-7-2-10(16)8-13(14)17/h2-9H,1H3,(H,18,19)
Clé InChI
OOLBCHYXZDXLDS-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
(+/–)-Diclofop is a chiral herbicide that belongs to the chemical class of chiral aryloxyphenoxypropionate compounds. The actual herbicidal active ingredient― carboxylic acid, is released after application through hydrolysis of the ester. It is absorbed mainly through leaves and inhibits the biosynthesis of fatty acids by suppressing the activity of acetyl CoA carboxylase (ACCase). It is used for the post-emergence control of wild oats, wild millets, and other annual grass weeds commonly occurring in wheat, barley, rye, red fescue, and broad-leaved crops.
It was included on 1st June 2011 in Annex I of Directive 91/414/EEC by the European Commission Directive 2011/45/EU. It is authorized for use under EC Regulation No 1107/2009, as per the Commission Implementing Regulation (EU) No 540/2011, however it is a candidate for substitution.
Application
The (+/–)-Diclofop CRM can also be used as following:
- To evaluate the likely enantioselective oxidative stress produced in Microcystis aeruginosa by diclofop acid
- For analyzing the phytotoxic effects of diclofop acid enantiomers on the plant Arabidopsis thaliana
- To study the enantioselective toxicity of diclofop acid on the non-target rice Xiushui 63 seedlings
- In the chiral separation of diclofop-acid using one- and two- dimensional HPLC methods
- To determine the enantioselective toxicity and degradation of diclofop in three algal cultures
Produits recommandés
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique