395242
(4S-trans)-2,2-Dimethyl-α,α,α′,α′-tetra(1-naphthyl)-1,3-dioxolane-4,5-dimethanol
99%
About This Item
Recommended Products
Assay
99%
form
solid
optical activity
[α]20/D +280°, c = 1 in ethyl acetate
mp
200 °C (dec.) (lit.)
functional group
ether
hydroxyl
ketal
SMILES string
CC1(C)O[C@@H]([C@H](O1)C(O)(c2cccc3ccccc23)c4cccc5ccccc45)C(O)(c6cccc7ccccc67)c8cccc9ccccc89
InChI
1S/C47H38O4/c1-45(2)50-43(46(48,39-27-11-19-31-15-3-7-23-35(31)39)40-28-12-20-32-16-4-8-24-36(32)40)44(51-45)47(49,41-29-13-21-33-17-5-9-25-37(33)41)42-30-14-22-34-18-6-10-26-38(34)42/h3-30,43-44,48-49H,1-2H3/t43-,44-/m0/s1
InChI key
WTZVNZRNIOJACO-CXNSMIOJSA-N
Application
- For the highly enantioselective addition of primary alkyl Grignards to ketones.
- As an organocatalyst for the activation of carbonyl functionality in vinylogous addition reaction of an aldehyde.
- As a chiral dopant in the preparation of cholesteric liquid crystal (CLC) having an aggregation-induced-emission dye.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The chiral auxiliaries TADDOLs (α,α,α,α-tetraaryl-1,3-dioxolane-4,5- dimethanols) developed by Seebach's group have found numerous applications in asymmetric synthesis ranging from utilization as stoichiometric chiral reagents or in Lewis acid mediated reactions, to roles in catalytic hydrogenation and stereoregular metathesis polymerization.
Chiral Diols
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 395242-1G | 4061826094860 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service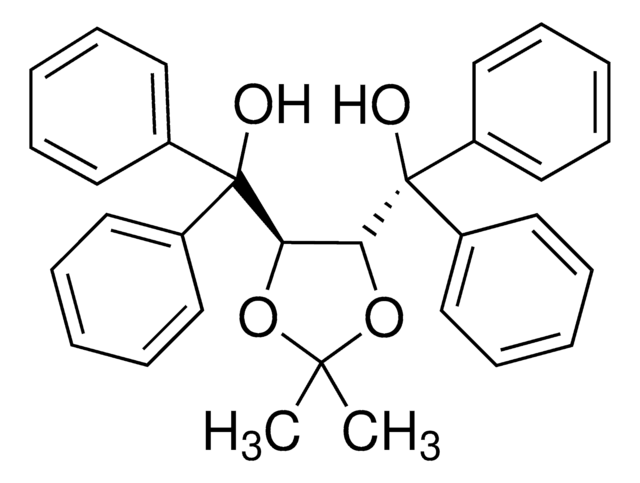
![Chlorocyclopentadienyl[(4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanolato]titanium 97%](/deepweb/assets/sigmaaldrich/product/structures/232/672/2ca86719-0965-4619-b25b-aae4087a2aad/640/2ca86719-0965-4619-b25b-aae4087a2aad.png)


![Zinc bis[bis(trimethylsilyl)amide] 97%](/deepweb/assets/sigmaaldrich/product/structures/294/819/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d/640/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d.png)