O-Linked Glycan Strategies
Glycobiology Analysis Manual, 2nd Edition
- O-Glycosidase
- Removal of Sialic Acid Residues
- Removal of β-Linked Galactose and N-Acetylglucosamine
- Other O-Linked Oligosaccharide Modifications
- Materials
Releasing O-linked glycans, or O-glycans, can involve various steps including removing different non-reducing end residues with exoglycosidases. Read on to learn about O-linked glycan strategies, such as the actions of O-glycosidase and how to remove di and trisialylation, β-linked galactose, and N-acetylglucosamine.
O-Glycosidase
While most N-linked oligosaccharides can be removed using PNGase F, there is no enzyme comparable to PNGase F for removing intact O-linked sugars. O-Glycosidase (also known as Endo-α-N-acetylgalactosaminidase or O-Glycanase) hydrolyzes the serine or threonine-linked unsubstituted O-glycan core [Gal-β(1→3)-GalNAc] (Figure 1). Any modification of the core structure can block the action of O-glycosidase. To avoid this issue, exoglycosidases like sialidases can be used to reduce the glycoform down to a core structure before or concurrently with the O-glycosidase treatment.
![O-Glycosidase Cleavage Site Diagram showing the cleavage site and structural requirements for O-Glycosidase which hydrolyzes the serine or threonine-linked unsubstituted O-glycan core [Gal-β(1→3)-GalNAc].](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/research-and-disease-areas/cell-signaling/o-glycosidase/o-glycosidase.jpg)
Figure 1.Cleavage site and structural requirements for O-Glycosidase. Any modification of the core structure will prevent cleavage.
Monosaccharides must be sequentially hydrolyzed by a series of exoglycosidases until only the Gal-β(1→3)-GalNAc core remains. O-Glycosidase (ex. G1163 or 324716) can then remove the intact core structure with no modification of the serine or threonine residues.1 Denaturation of the glycoprotein does not appear to significantly enhance O-deglycosylation.
Removal of Sialic Acid Residues
The most common modification of the core Gal-β(1→3)-GalNAc is a mono, di, or trisialylation.2,3,4,5 These sialic acid residues are easily removed by α-(2→3,6,8,9)-neuraminidase (ex. N8271) since only this enzyme is capable of efficient cleavage of the NeuAc-α(2→8)-NeuAc bond (Figure 2).6
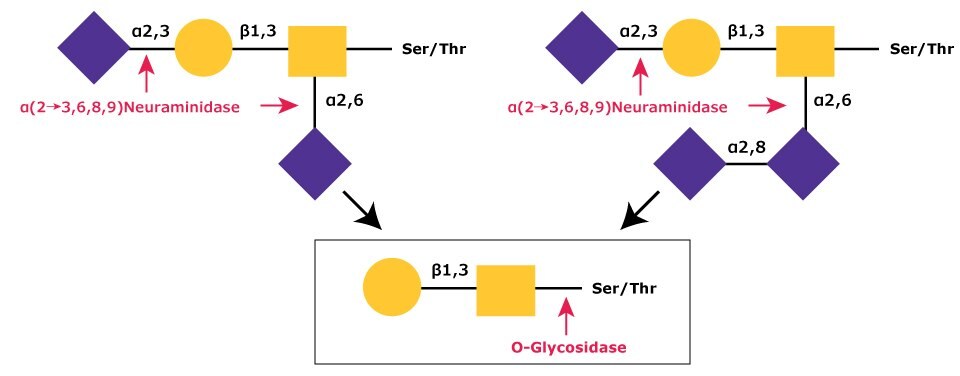
Figure 2.In sequential glycolytic cleavage, disialylated and trisialylated O-linked glycans have the sialic acid residues (NeuNAc) removed by α-(2→3,6,8,9)-neuraminidase. The Core 1 type glycan is then cleaved from the O-linkage by O-glycosidase.
Removal of β-Linked Galactose and N-Acetylglucosamine
Another commonly occurring O-linked hexasaccharide structure contains β(1→4)-linked galactose (Gal) and β(1→6)-linked N-acetylglucosamine (GlcNAc) as well as sialic acid.7,8 Hydrolysis of this glycan will require, in addition to neuraminidase, a β(1→4)-specific galactosidase (ex. G0413) and an N-acetylglucosaminidase (ex. A6805) (Figure 3). A non-specific galactosidase (ex. 345788 or G5635) will hydrolyze β(1→3)-galactose from the core glycan and leave an O-linked GalNAc residue that cannot be removed by O-glycosidase.

Figure 3.Disialylated O-linked Core 2 hexasaccharide is sequentially degraded by (1) removal of sialic acid residues (NeuNAc) using α(2→3,6,8,9) neuraminidase, (2) removal of β(1→4)-galactose (Gal) residues using β(1→4)-galactosidase, and (3) removal of N-acetylglucosamine (GlcNAc) using N-acetylglucosaminidase. The remaining Core 1 type glycan can then be cleaved from O-linkage using O-glycosidase as shown in Figure 2.
α-Neuraminidase (ex. N8271), β(1-4)-Galactosidase (ex. G0413), and β-N-Acetylglucosaminidase (ex. A6805) can be used for the hydrolysis of these and any other O-linked structures containing α-sialic acid, β(1-4)-linked galactose (Gal), or β-linked N-acetylglucosamine (GlcNAc) such as polylactosamine (Figure 4).

Figure 4.O-linked hexasaccharide structures containing β(1→4)-linked galactose (Gal) and β(1→6)-linked N-acetylglucosamine (GlcNAc) as well as di and trisialated O-linked glycans.
Other O-Linked Oligosaccharide Modifications
Less common modifications that have been found on O-linked oligosaccharides include α-linked galactose (Gal) and α-linked fucose.8.9 α-Gal has found to be a common allergen found in animal tissue, and can lead to meat allergies or xenotransplant immunogenicity. 10 A non-specific α-Galactosidase (ex. G8507) can be used to hydrolyze this type of linkage. N-acetylglucosamine attached directly to the peptide backbone (found on nuclear proteins) and α-linked N-acetylgalactosamine (found in mucins) have also been reported.11 Additional exoglycosidases are necessary for complete O-deglycosylation when these residues are present. Fucose and mannose directly O-linked to proteins cannot presently be removed enzymatically. 12,13
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?