TokeOni: Properties and Applications
Takahiro Kuchimaru, Shinae Kizaka-Kondoh
Graduate School of Bioengineering & Biotechnology, Tokyo Institute of Technology, Yokohama, Japan.
Introduction
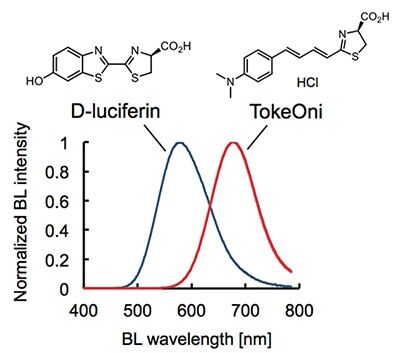
Bioluminescence imaging (BLI) systems allow for high-sensitive and noninvasive monitoring of cell proliferation, activity of signaling pathways and protein-protein interactions in living tissues.1-3 In this decade, BLI has extended its applications to a broad range of biomedical research. However, one bottleneck of the current BLI system is that the standard combination of firefly luciferase (Fluc) and its natural substrate D-luciferin emits a relatively short wavelength of bioluminescence (λmax = 560 nm) which is highly absorbed and scattered by biological tissues.4,5 To overcome this, a BLI system that utilizes near-infrared (NIR) bioluminescence has been desired.
TokeOni (Product No. 808350) is a first-time practical substrate that achieves 1) good chemical stability, 2) high water solubility and 3) robust emission of NIR-bioluminescence (λmax = 677 nm) with native Fluc. TokeOni is immediately compatible with current BLI systems using native Fluc and provides superior detection sensitivity of targets particularly in deep living tissues6.
High-sensitive imaging of deep tissues using TokeOni
TokeOni is dissolved in sterile water (not buffer solution) up to 40 mM of concentration and stored in -80 oC (< 3 months). TokeOni emits NIR-bioluminescence in reaction with native Fluc in the presence of ATP-Mg (Figure 1).
To exemplify the performance of TokeOni in vivo, detection sensitivity of bone metastasis was compared for D-luciferin with TokeOni. SCID mice were intra-arterially injected with human prostate cancer PC-3 stably expressing firefly luciferase (PC-3/luc) to form bone metastasis. 100 uL of the 33 mM substrate (〜50 mg/body) was intraperitoneally injected into the same mice with a 4-hour interval and bioluminescence images were acquired using IVIS-Spectrum (PerkinElmer). TokeOni greatly enhanced the signals emanating from metastatic lesions in the hindlimb and iliac bones, demonstrated by a 5.2-fold increase in the photon flux observed with TokeOni over D-luciferin (Figure 2).
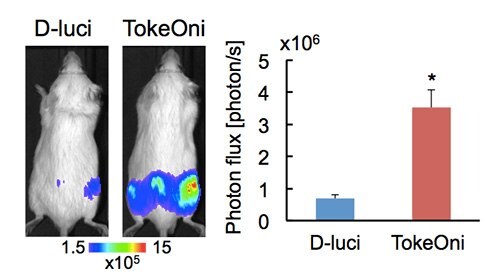
Figure 2.High-sensitive in vivo imaging of bone metastasis using TokeOni. Representative bioluminescence images obtained at 10 min after injecting the substrates (left panel) and quantitative analysis of bioluminescence intensity from hind limbs (right panel). TokeOni was injected into the same mice 4 hours after injecting D-luciferin. Error bars are standard error of the mean. n=6, *p<0.05.
TokeOni typically displays a peak bioluminescence production at a relatively early time point after the intraperitoneal injection compared to D-luciferin. Therefore, a peak-time bioluminescence production after i.p. injection of TokeOni will be found in the mouse model before performing in vivo imaging. In addition, the NIR-bioluminescence sensitivity to the luminometer has been enhanced when performing in vitro tube assays with TokeOni since typical luminometers for in vitro assays are less sensitive to the NIR light compared to the visible one.
Summary
TokeOni is one of the best options to observe fewer cells which could not be detected in deep tissue using D-luciferin. Improved detection sensitivity of TokeOni would allow for more accurate quantitative-detection of targets in vivo.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?