Assessing the Level of Bacteria in Purified Water
Stephane Mabic, Thomas Flint
Millipore S.A.S, Lab Water, Saint-Quentin-en-Yvelines, France
Abstract
This paper discusses the critical points for laboratories to consider when assessing bacteria levels in purified water and attempting to optimize the microbiology process. Good laboratory practices and procedures adapted to water purification systems are described for microbiology sampling, transportation, and culture steps.
Introduction
Assessing the level of bacteria in purified water is a common need in a variety of laboratories, including those in the pharmaceutical industry, biomedical sector, and environmental and testing companies. In order to achieve the correct microbiological count, attention should be given to a number of critical points, from the sampling procedure to the culture and counting conditions. Specific species of bacteria have been able to adapt to the stringent conditions of purified water, e.g., low nutrient conditions and osmotic pressure. The presence and adaptation of these heterotrophic bacteria species have been documented, and species such as Ralstonia pickettii, Sphingomonas paucimobilis, or Caulobacter crescensus are commonly identified in purified water.
Several aspects should be considered to optimize the microbiology process. These aspects have been described in pharmacopoeia and in the Clinical and Laboratory Standards Institute® (CLSI®) guideline1, for instance. In this application note, we present good laboratory practices and procedures, specifically adapted to purified water delivered by water purification systems, in order to ensure the optimization of the various steps in the microbiology process, namely sampling, transportation, and culture.
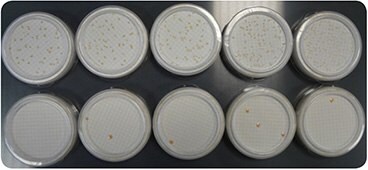
Figure 1.Comparison of bacteria growth on R2A (top) and TSA (bottom) media. The sample was split into ten portions of 100 mL, and five portions were grown on each medium. Conditions of incubations were: 72 h, 30 °C.
The bacterial count is completely erroneous on the TSA medium.
Sampling
Sampling is a key step in the microbiology process, and it must be done with extreme care. It is very important to flush the water purification system’s delivery point before collection, in order to completely rinse away the bacteria attached to the delivery valve or at the point of use. It is recommended to flush a minimum volume of two liters when water is collected from a final point-of-use filter, while up to five liters may be ideal when the delivery point is particularly sensitive to bacterial contamination (e.g., a ball valve on a storage tank or a laboratory environment favorable to bacterial contamination).
When the flushing is done carefully, it is not necessary to use ethanol to rinse the point of delivery. If ethanol is used, however, the sampling point needs to be thoroughly rinsed afterwards to ensure that no trace of alcohol is able to pass into the sample and kill bacteria. Using gloves during sampling is recommended to avoid external contamination.
Pure water should be collected in sterile containers and in volumes that allow a meaningful count. Samples of 250 mL are recommended whenever possible, which allow a duplicate analysis of a 100 mL volume. It should be noted that samples must be collected in a flow of water, without closing and opening valves at the time of sampling. Each sample must be carefully identified and labeled with sufficient information to ensure traceability.
Storage and Transportation
Sample storage should be reduced to a minimum before culture. If storage is unavoidable, it should be done in conditions allowing sample conservation and avoiding bacterial growth. Avoiding high temperatures is a key factor, and storage temperatures should not exceed 4 °C. A sample can be stored for two hours at room temperature or for 48 hours at 4 °C.
Transportation, if needed, must be carried out as soon as possible to avoid variations in the sample. Care should be taken when transporting samples during the summer months, and it might be necessary to ship samples in refrigerated containers to ensure that a low temperature is maintained until culture.
Culture
Cultures should be done on an appropriate growth medium. Indeed, bacteria in purified water are stressed by the poor conditions of their environment. Selected media, when combined with a low temperature and extended incubation times, will promote the growth of bacteria found in purified water. The most recommended medium is R2A, an agar-based medium. Medium S can also be used. Media such as TSA or chocolate blood agar are not appropriate (Figure 1). Temperatures of 25 to 30 °C are commonly used to grow bacteria in purified water, with incubation times up to five days, to allow slow-growing bacteria strains to develop.
A very common way to process the sample is filtration through a membrane (< 0.45 μm) using a vacuum pump, and then transferring the membrane directly onto the R2A culture medium (e.g. Milliflex® Rapid system). This semi-automated process reduces the risk of contamination of the sample.

Figure 2.Steps to assess the level of bacteria in purified water
Conclusion
Following these steps and advice throughout the microbiology process reduces the risks of contamination and the risks of false positive and erroneous bacterial counts. This approach is fully in line with good practices recommended by the European, US, or Japanese Pharmacopoeia, as well as other global regulatory organizations.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?