Passaging Human iPS Cells Protocol Using EZ-LiFT™ Reagent
A common problem associated with passaging human pluripotent stem cells, including ES and iPS cells, is the unwanted spontaneous differentiation of cells. Without manual intervention, differentiated cells proliferate quickly and eventually diminish the overall quality of the stem cell culture. EZ-LiFT™ Stem Cell Passaging Reagent is a proprietary enzyme-free and chemically defined stem cell dissociation reagent that selectively passages only undifferentiated pluripotent stem cells. The reagent eliminates the need for manual colony selection or cell scraping and produces high cell viability without the need for ROCKi. EZ-LiFT™ can also rescue highly differentiated iPS cell cultures. The reagent is time dependent and the following detailed protocol is recommended for successful passaging of human iPS cells.
Human iPSC Passaging Protocol
Important Notes: Parameters important to maintain high quality pluripotent cultures are outlined below.
- Incubation time: Colony dissociation is time dependent. To ensure high quality, passage no more than 3 wells at a time. The protocols outlined below are based on passaging one well of a 6-well plate.
- Colony confluence: The optimal cell confluence is 60-80% confluency. However, overconfluent cultures can still be rescued by longer incubation time with EZ-LiFT™ Reagent (see protocol below).
- Temperature: For the most consistent results, we recommend that the EZ-LiFT™ Reagent and the shaker (if used) be warmed to 37 °C before starting. Minimize the time that the cells are not at 37 °C. Do not rinse your cell culture with any ice-cold solution or medium before treatment with EZ-LiFT™ as the cold temperature may slow down the rate of colony dissociation. If cells are washed with ice cold medium, re-incubate culture at 37 °C for at least 30 minutes before applying EZ-LiFT™ Reagent.
- Shaking speed: The protocol was optimized using a Labnet VorTemp 56 orbital shaker with 500 rpm as the optimal speed. If using another shaker, optimal speed may need to be determined.
- Do not over-pipette. After treatment with EZ-LiFT™, detached clumps are fragile and can easily break apart into single cells.
Passaging Without a Shaker:
- Start with high-quality human ES or iPS cell culture that is 60-80% confluent. Warm EZ-LiFT™ Reagent (SCM139) to 37 °C before starting.
Critical Note: Do not use ice-cold EZ-LiFT™ Reagent as this will slow down the colony dissociation. - Aspirate the culture medium and wash wells twice with 1.5 mL EZ-LiFT™ Reagent (SCM139). Aspirate after each wash.
- Add 1 mL of EZ-LiFT™ reagent (SCM139) to each well. Incubate the plate at 37 °C for 4 minutes.
- After 4 minutes, tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
- Place the plate back in the 37 °C incubator for an additional 4 minutes.
- After 4 minutes, tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
Important: Do not rinse the wells. - Perform a quick microscopic inspection of the well(s).
a. If a significant number of detached clumps are visible, proceed to step 8.
b. If no obvious detachment is observed, repeat steps 4-7 except that in step 5, the 37°C incubation should be for 2 instead of 4 minutes. Proceed to step 8. - Gently collect the cell suspension (~1 mL) and transfer to a 15 mL conical tube. Neutralize with 5 mL of culture medium by gently adding the medium to the cell suspension. Do not pipette up and down as this may break cell clumps into single cells suspension.
- Centrifuge at 800 rpm or 130 x g for 3 minutes. Aspirate the supernatant.
- Gently resuspend the cell pellet in 1 mL pluripotent medium such as PluriSTEM™ Human ES/IPS Media (SCM130).
Caution: Do not pipette up and down more than two times. Over-pipetting may result in single cell dissociation. - Passage dissociated cell clumps to newly coated 6 well plates. If you are a first time user, we recommend passaging at a conservative 1:5 split ratio. After becoming familiar with the protocol, the split ratio may be increased to 1:10 up to 1:30 split ratio. Monitor cells daily. Newly passaged ES/iPS cells will typically reach 60-80% confluence in 6-8 days depending upon the split ratio.
Passaging Using a Shaker:
- Start with high-quality human ES or iPS cell culture that is 60-80% confluent. Warm EZ-LiFT™ Reagent (SCM139) to 37 °C before starting.
Critical Note: Do not use ice-cold EZ-LiFT™ Reagent as this will slow down the colony dissociation. - Place a Labnet VorTemp™ 56 orbital shaker into the 37 °C incubator. Be sure to wipe down the shaker with 70% ethanol first.
- Aspirate the culture medium and wash wells twice with 1.5 mL EZ-LiFT™ Reagent (SCM139). Aspirate after each wash.
- Add 1 mL of EZ-LiFT™ reagent (SCM139) to each well.
- Seal the plate with paraffin to prevent cross-contamination while shaking.
- Shake plate at 500 rpm for 5 minutes at 37 °C.
- After 5 minutes, tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
Important: Do not rinse the wells. - Perform a quick microscopic inspection of each well.
a. If a significant number of detached clumps are visible, proceed to step 9.
b. If no obvious detachment is observed:
1. Tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
2. Shake for 1 minute
3. Tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs). Procced to Step 9. - Gently collect the cell suspension (~1 mL) and transfer to a 15 mL conical tube. Neutralize with 5 mL of culture medium by gently adding the medium to the cell suspension. Do not pipette up and down as this may break cell clumps into single cells suspension.
- Centrifuge at 800 rpm or 130 x g for 3 minutes. Aspirate the supernatant.
- Gently resuspend the cell pellet in 1 mL pluripotent medium such as PluriSTEM™ Human ES/IPS Media (SCM130).
Caution: Do not pipette up and down more than two times. Over-pipetting may result in single cell dissociation. - Passage dissociated cell clumps to newly coated 6 well plates. If you are a first time user, we recommend passaging at a conservative 1:5 split ratio. After becoming familiar with the protocol, the split ratio may be increased to 1:9 up to 1:30 split ratio. Monitor cells daily. Newly passaged ES/iPS cells will typically reach 60-80% confluence in 6-8 days depending upon the split ratio.
Cell Cryopreservation
- Start with high-quality human ES or iPS cell culture that is 60-80% confluent. Warm EZ-LiFT™ Reagent (SCM139) to 37 °C before starting.
- Treat wells with EZ-LiFT™ (SCM139) as outlined in the protocols above. However instead of passaging to newly coated 6-well plates, resuspend cell pellet in 1 mL pluripotent media such as PluriSTEM™ Human ES/IPS Media (SCM130) containing 10% DMSO (D2650).
Caution: Do not pipette up and down more than two times. Over-pipetting may result in single cell dissociation. - One well of EZ-LiFT™ treated cells may be frozen into 3-5 cryovials. Depending upon the desired number of cryovials, add the requisite volume of pluripotent media containing 10% DMSO. For example, to obtain 5 cryovials, add 4 mL pluripotent media containing 10% DMSO to the 1 mL cell suspension in step 2.
- Transfer cryovials to Nalgene slow freeze Mr. Frosty container and store at -80 °C.
- After 24 hours, transfer cryovials to liquid nitrogen for long-term storage.
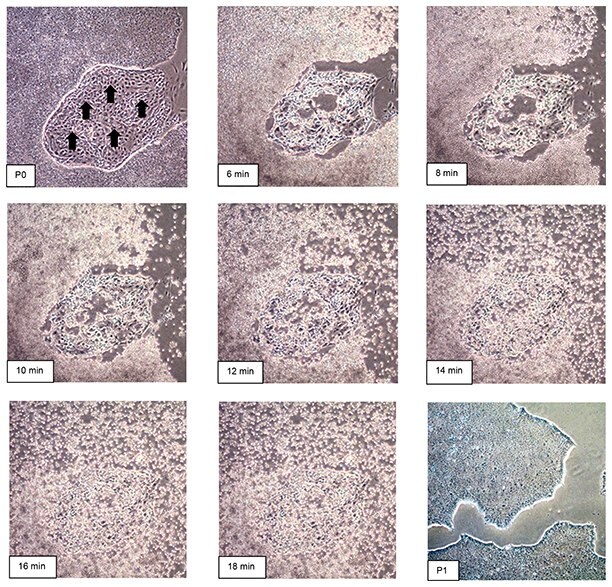
Figure 1.Time course of human iPS cell passaging using EZ-LiFT™ reagent. Incubation of cells with EZ-LiFT™ reagent was monitored for 18 minutes to illustrate the effects of EZ-LiFT™ on stem cell detachment. Human iPSCs before passaging with EZ-LiFT™ reagent contain areas of unwanted spontaneous differentiation (Arrows, P0). After 6 minutes of incubation time with EZ-LiFT™ reagent, very little colony detachment was observed. Significant amounts of detached cells was observed after 8-12 minutes. Thus, the optimal incubation time of EZ-LiFT™ Reagent for this human iPS culture was determined to be 10 minutes. Over incubation with EZ-LiFT™ reagent (14-18 minutes) results in detachment of all cells including differentiated subtypes. One passage after optimized EZ-LiFT™ iPSC passaging results in undifferentiated cell phenotype (P1).

Figure 2.EZ-LiFT™ reagent removes feeders from human iPSC cocultures. Human ips cells cultured on a mef feeder layer (top row) are selectively detached and passaged using EZ-LiFT™ reagent. 48 hours after passaging, feeder cells are absent while remaining iPSCs retain a pluripotent phenotype (bottom row).
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?