L-030
Lurasidone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Empfohlene Produkte
Qualität
certified reference material
Qualitätsniveau
Form
liquid
Leistungsmerkmale
Snap-N-Spike®/Snap-N-Shoot®
Verpackung
ampule of 1 mL
Hersteller/Markenname
Cerilliant®
Konzentration
1.0 mg/mL in methanol (as free base)
Methode(n)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Anwendung(en)
clinical testing
Format
single component solution
Lagertemp.
−20°C
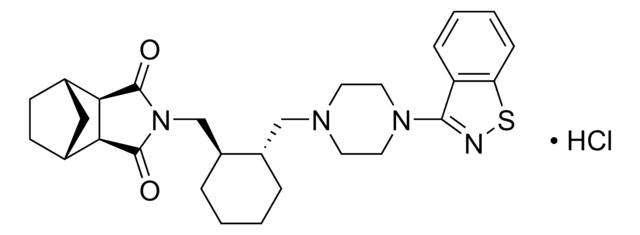
SMILES String
Cl.O=C1[C@@H]2[C@H]3CC[C@H](C3)[C@@H]2C(=O)N1C[C@@H]4CCCC[C@H]4CN5CCN(CC5)c6nsc7ccccc67
InChI
1S/C28H36N4O2S.ClH/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26;/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2;1H/t18-,19+,20-,21-,24+,25-;/m0./s1
InChIKey
NEKCRUIRPWNMLK-SCIYSFAVSA-N
Angaben zum Gen
human ... DRD2(1813) , HTR2A(3356)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- LurasiDonein Bipolar Depression Research: A study explored the pharmacodynamic properties of lurasidone, hypothesizing its efficacy in acute bipolar depression. This research provides a deep dive into the mechanistic actions of lurasidone, enhancing understanding in neuropharmacological studies and aiding in the development of more effective treatments for bipolar disorder (Fountoulakis et al., 2024).
- Quantification of LurasiDonein Clinical Samples: Development and validation of a liquid chromatography-tandem mass spectrometry method for quantifying lurasiDonein dried blood spot samples was reported. This method facilitates easier and less invasive monitoring of lurasiDonelevels in patients, crucial for effective pharmacological research and ensuring therapeutic efficacy in treatment regimes (Rajadhyaksha and Londhe, 2023).
- Novel Methodologies in Clinical Trials: Research introduced a novel method for deriving adverse event prevalence in randomized controlled trials, which could potentially improve the understanding of the benefit-risk ratio of drugs including lurasidone. This approach is particularly relevant for drug labels and regulatory submissions, ensuring safer and more effective clinical outcomes (Piacentino et al., 2024).
- Pharmacological Properties of Lurasidone: A study investigated how lurasiDoneblocks the voltage-gated potassium channels of coronary arterial smooth muscle cells, offering insights into its broader pharmacological impacts. This research is vital for assessing potential cardiovascular side effects and optimizing dosing strategies to mitigate risks in patients treated with lurasiDone(Zhuang et al., 2023).
Rechtliche Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Zielorgane
Eyes
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 1
Flammpunkt (°F)
49.5 °F - closed cup
Flammpunkt (°C)
9.7 °C - closed cup
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.