Magnetic Properties of Materials
Magnetic Properties of Materials
Magnetic properties other than diamagnetism, which is present in all substances, arise from the interactions of unpaired electrons. These properties are traditionally found in transition metals, lanthanides, and their compounds due to the unpaired d and f electrons on the metal. There are three general types of magnetic behaviors: paramagnetism, in which the unpaired electrons are randomly arranged, ferromagnetism, in which the unpaired electrons are all aligned, and antiferromagnetism, in which the unpaired electrons line up opposite of one another. Ferromagnetic materials have an overall magnetic moment, whereas antiferromagnetic materials have a magnetic moment of zero. A compound is defined as being ferrimagnetic if the electron spins are orientated antiparrallel to one another but, due to an inequality in the number of spins in each orientation, there exists an overall magnetic moment. There are also enforced ferromagnetic substances (called spin-glass-like) in which antiferromagnetic materials have pockets of aligned spins (Figure 1).
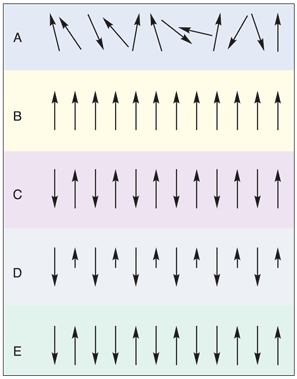
Figure 1.Types of magnetism: (A) paramagnetism (B) ferromagnetism (C) antiferromagnetism (D) ferrimagnetism (E) enforced ferromagnetism
Magnetic character of materials is typically analyzed relative to its magnetic susceptibility (χ). Magnetic susceptibility is the ratio of magnetization (M) to magnetic field (H). The type of magnetic behavior of a compound can be defined by its value of χ (Table 1 for a comparison of magnetic behavior versus χ and Table 2 for the susceptibilities of some common paramagnetic materials).
| Magnetic Behavior | Value of χ |
|---|---|
| Diamagnetic | small and negative |
| Paramagnetic | small and positive |
| Ferromagnetic | large and positive |
| Antiferromagnetic | small and positive |
| Compound/ Element | Formula | Mass Susceptibility (χm) (m3/kg) | Mass Susceptibility (χm) (emu/Oe•g) x 10-3 |
|---|---|---|---|
| Cerium | Ce | 64.84 | 5.160 |
| Chromium(III) oxide | Cr2O3 | 24.63 | 1.960 |
| Cobalt(II) oxide | CoO | 61.57 | 4.900 |
| Dysprosium | Dy | 1301 | 103.500 |
| Dysprosium oxide | Dy2O3 | 1126 | 89.600 |
| Erbium | Er | 556.7 | 44.300 |
| Erbium oxide | Er2O3 | 928.9 | 73.920 |
| Europium | Eu | 427.3 | 34.000 |
| Europium oxide | Eu2O3 | 126.9 | 10.100 |
| Gadolinium | Gd | 9488 | 755.000 |
| Gadolinium oxide | Gd2O3 | 668.5 | 53.200 |
| Iron(II) oxide | FeO | 90.48 | 7.200 |
| Iron(III) oxide | Fe2O3 | 45.06 | 3.586 |
| Iron(II) sulfide | FeS | 13.5 | 1.074 |
| Neodymium | Nd | 70.72 | 5.628 |
| Neodymium oxide | Nd2O3 | 128.2 | 10.200 |
| Potassium superoxide | KO2 | 40.59 | 3.230 |
| Praseodymium | Pr | 62.96 | 5.010 |
| Samarium | Sm | 28.02 | 2.230 |
| Samarium oxide | Sm2O3 | 24.98 | 1.988 |
| Terbium | Tb | 1822 | 146.000 |
| Terbium oxide | Tb2O3 | 984.4 | 78.340 |
| Thulium | Tm | 320.4 | 25.500 |
| Thulium oxide | Tm2O3 | 646.5 | 51.444 |
| Vanadium oxide | V2O3 | 24.83 | 1.976 |
Antiferromagnetic materials can be distinguished from paramagnetic substances in that the value of χ increases with temperature, whereas χ shows no change or decreases in value as temperature rises for paramagnetic compounds. Ferromagnetic and antiferromagnetic materials will lose magnetic character and become paramagnetic if sufficiently heated. The temperature at which this occurs is defined as the Curie temperature (Tc) for ferromagnetic compounds and the Néel temperature (TN) for antiferromagnetic compounds. Some substances, particularly the later lanthanides, will go from paramagnetic to antiferromagnetic to ferromagnetic as temperature decreases (Table 3).
| Curie Temperature | Néel Temperature | urie Temperature | Néel Temperature | |
|---|---|---|---|---|
| Metal | TC (°C) | TN (°C) | TC (K) | TN (K) |
| Ce | -260.65 | 12.5 | ||
| Pr | -248 | 25 | ||
| Nd | -254 | 19 | ||
| Sm | -258.35 | 14.8 | ||
| Eu | -183 | 90 | ||
| Gd | 20 | 293 | ||
| Tb | -51 | -44 | 222 | 229 |
| Dy | -188 | -94 | 85 | 179 |
| Ho | -253 | -142 | 20 | 131 |
| Er | -253 | -189 | 20 | 84 |
| Tm | -248 | -217 | 25 | 56 |
There are several unique properties of magnetic materials which are exploited. Changing magnetic fields induce an electrical voltage making magnetic materials a central component of nearly all electrical generators. Magnetic materials are also essential components for information storage in computers, sensors, actuators, and a variety of telecommunications devices ranging from telephones to satellites.
Some materials, known as soft magnetic materials, exhibit magnetic properties only when they are exposed to a magnetizing force such as a changing electric field. Soft ferromagnetic materials are the most common of these as they are widely used in both AC and DC circuits to amplify the electrical flux. Magnetic nanopowders have shown great promise in advanced soft magnetic materials.2 Magnetocaloric materials heat up in the presence of a magnetic field and subsequently cool down when removed from the magnetic field. Pure iron, for example will change temperature by 0.5 – 2.0 °C/Tesla. More recently alloys of the formula Gd5SixGe1-x (where x = 0 – 5) will exhibit a 3 – 4 °C/Tesla change.3,4 Some nanomagnetic materials have shown significant magnetocaloric properties.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?