Peptide Resin Loading Protocols
1. Merrifield resins
Merrifield resin (Product No. 497061, 474517) can be loaded with Boc-amino acids as described in Method 1,1 or can be purchased pre-loaded with the C-terminal amino acid.

Method 1. Attachment of Boc-amino acids to Merrifield resins
- Dissolve Boc-amino acid in EtOH (2 mL/mmol) and add water (0.5 mL/mmol). Adjust pH to 7 with 2 M aq. Cs2CO3. Evaporate solution to dryness. Add dioxane and evaporate to dryness. Repeat evaporation with dioxane.
- Pre-swell Merrifield resin in DCM for 1 h and then wash with DMF. Add Cs salt (1.2 eq.) in DMF to the resin and heat at 50 °C o/n. The reaction may be catalyzed by the addition of KI (0.1 eq.). At the end of this time, wash the resin with 3X DMF, 3X DMF/water (1:1), 3X DMF, 3X DCM, 3x MeOH. Dry in vacuo over KOH.
2. Hydroxymethyl- functionalized resins
One of the simplest methods for esterification to hydroxymethyl-functionalized linkers (Wang, HMPA, and HMBA resins) is to use the symmetrical anhydride of the protected amino acid in the presence of a catalytic amount of p-dimethylaminopyridine (DMAP) (Method 2).2 However, due to the basic character of this material, enantiomerization and dipeptide formation can be expected; the amount depending on the quantity of DMAP used, the length of the reaction and the nature of the amino acid.

The MSNT method3,4 (Method 3) is the method of choice in difficult circumstances, such as loading of HMBA resins or when attaching enantiomerization prone amino acid derivatives.5,6
Cysteine and histidine are particularly prone to enantiomerization and should not be loaded by this method. For these residues, the use of 2-ClTrt resin is recommended; esterification of the C terminal residue is free from enantiomerization and dipeptide formation7 because attachment does not involve activation of the incoming protected amino acid (Method 5). When peptide acids containing Pro as the C-terminal residue are desired, the use of trityl-based resins is recommended.
Once the resin is loaded the substitution of the resin can be easily determined using Method 10.
Method 2: Attachment to hydroxymethyl resins using symmetrical anhydride
- Place the resin (1 g) in a clean, dry flask, and add sufficient DMF to just cover and allow to swell for 30 min. Add extra DMF if necessary just to cover the resin.
- Dissolve the Fmoc amino acid (10 eq. relative to resin loading) in dry DCM. One or two drops of DMF may be needed to aid complete dissolution.
- Add a solution of diisopropylcarbodiimide (5 eq. relative to resin loading) in dry DCM to the amino acid solution.
- Stir the mixture for 20 min at 0 °C, keeping the reaction mixture free of moisture with a calcium chloride drying tube.
- Remove the DCM by evaporation under reduced pressure using a rotary evaporator.
- Dissolve the residue in the minimum of DMF and add the solution to the resin prepared in step 1.
- Dissolve DMAP (0.1 eq. relative to resin loading) in DMF and add this solution to the resin/amino acid mixture. Stopper the flask and allow the mixture to stand at rt for 1 h with occasional swirling.
- Remove a small sample of resin (20 mg) and wash, dry and estimate the level of first residue attachment using the procedure described in Method 10. If the value obtained is less than 70% the first residue attachment procedure should be repeated.
Note: This method is not suitable for His or Cys.
Method 3: Attachment to hydroxymethyl resins using MSNT/MeIm
- Place the resin in a dry reaction vessel. Swell and wash with DCM, add sufficient DCM to cover resin and flush vessel with nitrogen.
- Weigh the appropriate Fmoc amino acid (5 eq.) into a dry round bottom flask. Add dry DCM to dissolve the amino acid derivative (approximately 3 mL/mmol); one or two drops of THF can be added to aid dissolution.
- Add MeIm (3.75 eq.) followed by MSNT (5eq.). Flush flask with nitrogen and seal. Stir the mixture until the MSNT has dissolved.
- Using a syringe, transfer the amino acid solution to the vessel containing the resin.
- Allow the mixture to stand at rt for 1 h, with gentle agitation.
- Wash with DCM (5 times) and DMF (5 times).
- Remove a small sample of resin (20 mg) and wash, dry and estimate the level of first residue attachment using the procedure described in Method 10. If the value obtained is less than 70% the first residue attachment procedure should be repeated.
3. Loading amino- functionalized resins
Attachment of amino acid derivatives and other carboxylic acids to linkers containing primary amino groups can normally be affected using standard methods of amide bond formation (see Method 4). Hydroxylamine, Weinreb amide, and resins functionalized with secondary amines are much more difficult to load; for these the use of HOAt/DIPCDI or HATU/DIPEA activation is required.
Method 4: Attachment of carboxylic acids to amino resins
- Pre-swell the resin with DCM (polystyrene-based resins) or DMF (PEG-PS or PEGA resin) for 1 h. Wash resin thoroughly with DMF.
- If the resin is Fmoc protected, treat with 20% piperidine in DMF for 20 min, then wash resin thoroughly with DMF.
a) Rink Amide, Sieber Amide, MBHA
- Dissolve amino acid derivative or carboxylic acid (5 eq.) and HOBt in DMF. Add DIPCDI (5 eq.) and leave to stand for 10 min. Add mixture to resin. Leave for 1-6 h.
b) N-Alkylamino resins
- Dissolve amino acid derivative or carboxylic acid (5 eq.) and HATU in DMF. Add DIPEA (10 eq.) and add immediately to resin. Leave for 6 h.
Remove a small quantity of resin and test this for the presence of unreacted amines using the TNBS test (primary amine resins) or chloranil test (secondary amine resins). If the result is positive, wash the resin with DMF and repeat coupling. Continue this procedure until a negative result is obtained.
4. Trityl-based resins
In contrast to benzyl alcohol-based supports, attachment of Fmoc-amino acids to trityl-based resins, such as 2-chlorotrityl or NovaSyn® TGT resins, is free from enantiomerization,7 making them ideal for the immobilization of sensitive residues such as Cys and His (Method 5). The resin also protects Cys from enantiomerization during chain extension. They are particularly useful in the synthesis of C-terminal prolyl peptides as the bulk of the trityl linker helps to prevent diketopiperazine formation8-10. When loading 2-chlorotrityl chloride resin, it is important to ensure that all amino-acid derivatives, glassware and solvent are thoroughly dried before use.
NovaSyn® TGT alcohol resins must be converted to the chloride form before attachment of the amino acid (Method 6).

Method 5: Loading of trityl resins
NOTE: it is important to dry all solvents and glassware before use.
Attachment of carboxylic acids
- Dissolve the carboxylic acid (0.6-1.2 eq. relative to the resin for 2-chlorotrityl resin and 2 eq. for NovaSyn® TGT chloride resin) and DIPEA (4 eq. relative to carboxylic acid) in dry DCM (approx. 10 ml per gram of resin) containing, if necessary, a small amount of dry DMF (just enough to facilitate dissolution of the acid). For pseudoproline dipeptides add 3 mL of NMP/gram of resin.
- Add this to the resin and stir for 30-120 min. For pseudoproline dipeptides leave to react o/n. At the end of this time, wash the resin with 3X DCM/MeOH/DIPEA (17:2:1), 3X DCM; 2X DMF, 2X DCM. Dry in vacuo over KOH. Fmoc-amino acids are best dried before use by repeated evaporation from dioxane; determine loading using Method 10.
Method 6: Chloridation of NovaSyn® TGT alcohol resin
NOTE: it is important to dry all solvents and glassware before use.
- Place NovaSyn® TGT alcohol resin in a sintered glass funnel and wash the resin consecutively with DMF (2X), dry DCM (3X) and dry toluene (3X).
- Drain off excess toluene from the resin and transfer damp material to a round bottom flask equipped with a reflux condenser.
- Add sufficient toluene to cover resin, then add freshly distilled AcCl (1 mL/g of resin). Heat at 60 - 70 °C for 3 h.
- Slurry mixture to a sintered glass funnel. Wash resin with dry toluene (3X) and dry DCM (3x).
- Drain excess solvent from resin and use immediately.
5. Dbz resins
Success in using the Dbz strategy11 depends on regioselective acylation of only one of the two linker amines with the C-terminal amino acid residue and to avoid acylation of the unprotected amine during chain extension. Incomplete acylation leads to formation of C-terminally truncated peptides as new chains are propagated by acylation of any unreacted amines during subsequent coupling cycles. Whereas, overacylation results to formation of branched peptides with chains growing off both linker amines. Therefore, the selection of acylation method of attachment of the C-terminal residue and subsequent couplings is critical for good results (Method 7).
Method 7: Loading of Dbz resins
- Pre-swell the resin (0.1 mmol) in DCM for 60 min and wash with DMF. Remove Fmoc group with 20% piperidine in DMF and wash with DMF.
- Ile, Val, Thr, Pro, Arg: Add Fmoc-Aaa-OH (0.6 mmol), HATU (0.6 mmol) and DIPEA (0.9 mmol). Agitate gently for 1 h. Wash resin with DMF and repeat coupling.
- Gly: Add Fmoc-Gly-OPfp (0.6 mmol) and HOBt (0.6 mmol). Agitate gently for 1 h
- Other amino acids: Add Fmoc-Aaa-OH (0.6 mmol), HCTU (0.6 mmol) and DIPEA (0.9 mmol). Agitate gently for 1 h.
- Check loading using Method 10. Alternatively, wash a sample of resin with DCM and treat with 95% TFA aq. for 30 min. Anaylze cleaved product by HPLC.
Particularly problematic is the coupling of glycine residues, especially if they occur close to the C-terminus of the peptide. This reactive and unhindered amino acid can couple to the free Dbz-amino group if uronium or phosphonium activation is used. In our hands best results are obtained if glycine residues are introduced using Fmoc-Gly-OPfp/HOBt. This precaution may be unnecessary once the peptide is extended beyond 10 residues, as hindrance should reduce the reactivity of unprotected Dbz amine. Furthermore, the use of strong activators like HATU or HCTU should be avoided as their use can lead to branching. In our hands, HBTU/HOBt appears to work well for coupling of all residues except Gly, where the use of the pre-formed OPfp in conjunction with HOBt gives minimal branching.
The use of Alloc protection for blocking the second amino group has been advocated to avoid all issues with branching and truncation12. Dbz resins as supplied contain mostly 3-Fmoc-Dbz, with small amounts of 4-Fmoc-Dbz and bis-Fmoc-Dbz. Capping the resin with Alloc-Cl prior to removal of the Fmoc group will thus reduce the maximum potential for branching or truncation to 6%. For hindered amino acids, it has been found necessary to load the resin prior to capping with Alloc. The Alloc group must be cleaved off with Pd(0) before conversion to the Nbz form (Figure 1).
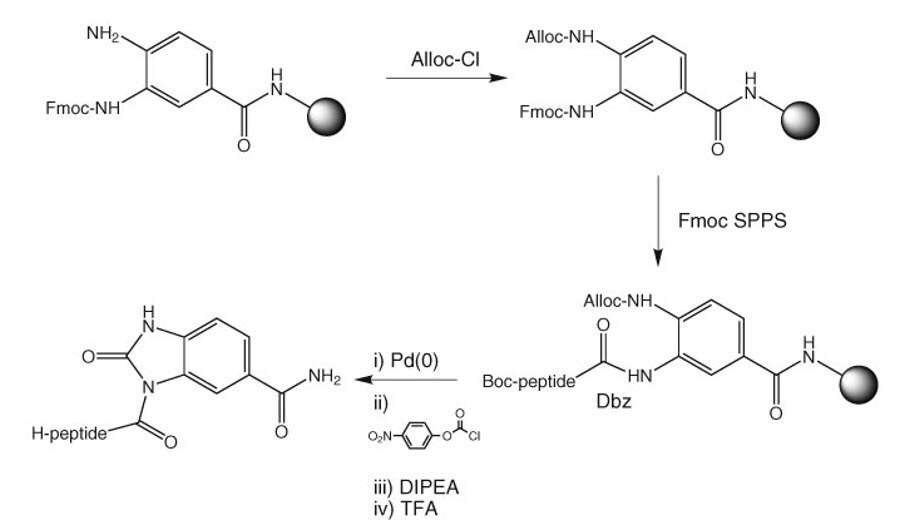
Figure 1: Alloc protection to avoid branching in Dbz resin
6. Sulfamyl resins
Loading of sulfamyl-based resins is best achieved with carboxylic acids activated with PyBOP® and DIPEA in CHCl3 at -20 °C13 or with DIPCDI/N-methylimidazole (Method 8). In the case of PyBOP® activation, the loading efficiencies are reported to vary from >95% for Cys, Met and His to 44% for Pro, the worst case. Extent of racemization for the loading of Fmoc-Phe and Fmoc-Leu by these methods are 0.5% and 0.3%, respectively. However, in practice the loading obtained by these methods can be highly variable, and problems can occur with over acylation of the linker. Furthermore, the substitution of the support must be determined before starting peptide synthesis.

Method 8: Loading of sulfamyl resins
DIPCDI method
- Pre-swell resin (1 mmol) in DCM for 1 h before use.
- Dissolve amino acid derivative or carboxylic acid (4 mmol) and 1-MeIm (4 mmol) in DCM/DMF (4:1). Add DIPCDI (4 mmol), mix and add to resin. Leave for stand with gentle agitation for 18 h. Wash resin with DMF, DCM, MeOH and dry.
PyBOP® method
- Pre-swell resin (1 mmol) in CHCl3 for 1 h before use.
- Dissolve amino acid derivative or carboxylic acid (4 mmol) and DIPEA (8 mmol) in CHCl3. Add to resin.
- Cool mixture to –20 °C. Add PyBOP® (4 mmol) and leave for stand with gentle agitation for 8 h at –20 °C. Wash resin with DCM, DMF, DCM, MeOH and dry.
7. DHP resins
DHP HM resin consists of 3,4-dihydro-2H-pyran-2-yl-methanol linker14 attached to 100-200 mesh chloromethyl polystyrene, and is a useful tool for the synthesis of peptide alcohols.
In contrast to trityl-based supports, where the use of prolonged reaction times and elevated temperatures are often required to achieve satisfactory loadings, derivatization of DHP HM resin is relatively straightforward, with even secondary alcohols being loaded without difficulty. Typically, this process involves treating the resin in DCE with an excess of alcohol in the presence of pyridinium p-toluenesulfonate (PPTS); full experimental details are given in Method 9.
Method 9: Loading DHP HM resin
- Pre-swell DHP HM resin in dry DCE for 1 h.
- Dissolve Fmoc-amino alcohol (3 eq.) in dry DCE containing PPTS (1.5 eq), and add this solution to the resin.
- Leave to react o/n at 80 °C with gentle agitation under nitrogen.
- Quench reaction by adding pyridine (~ 5 mL/g). Isolate resin by filtration and wash with DMF, DCM and hexane. Dry resin o/n under vacuum.
8. Preparation of peptide aldehydes using H-Thr-Gly-NovaSyn® TG resin
One of the simplest and most effective methods of preparing peptide aldehydes, which involves the solid-phase immobilization of an amino aldehyde by formation of an oxazolidine between a pre-formed Fmoc-amino aldehyde and H-Thr-Gly-NovaSyn® TG resin15.

After loading the resin, the oxazolidine nitrogen should be blocked by treatment with Boc-anhydride. The resultant acyloxazolidine is stable to base and is compatible with Fmoc protocols.
For peptides containing a C-terminal aspartal, argininal, leucinal, phenylalaninal, or valinal residue, pre-loaded resins are available.
Method 10: Loading of H-Thr-Gly-NovaSyn® TG resin
- Suspend H-Thr-Gly-NovaSyn® TG resin in 1% AcOH in MeOH/DCM (1:1) containing Fmoc-amino aldehyde (5 eq. relative to resin substitution) in DCM.
- Gently agitate mixture at rt for 4 h and monitor by TNBS test.
- Remove resin by filtration, wash with DCM, DMF, and THF.
- Treat resin with Boc2O (5 eq.) and NMM (5eq.) in THF at 50°C for 3 h to cap oxazolidine nitrogen.
- Remove resin by filtration and wash with THF, DCM, and DMF
10. Fmoc loading test
For estimating the loading of resins derivatized with Fmoc-amino acids, the simplest approach involves cleaving the Fmoc group with DBU and measuring the solution concentration of the liberated dibenzofluvene by U.V. spectroscopy.16
Method 11: Estimation of level of first residue attachment
- Take 3 x 10 mm matched silica UV cells.
- Weigh dry Fmoc amino acid-resin (approx. 5 µmol with respect to Fmoc) into a10 mL graduated flask.
- Add 2 mL of 2% DBU in DMF. Agitate gently for 30 min. Dilute solution to 10 mL with MeCN. Take 2 mL of this solution and dilute to 25 mL in a graduated flask
- Prepare a reference solution as in step 2, but without addition of the resin.
- Fill two cuvettes with 3 mL of test solution and one cuvette with 3 mL of reference solution. NOTE: Do not cross-contaminate the solutions. Allow the resin to settle to the bottom of the cells.
- Place the cells in a spectrophotometer and record optical density at 304 nm.
- Obtain an estimate of first residue attachment from equation below Fmoc loading: mmol/g =(Abssample-Absref) X 16.4/mg of resin).
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?