The Pd-catalyzed C–N bond formation has become an important synthetic reaction in the past 20 years. Several research groups have investigated this reaction and developed very versatile catalysts. Buchwald and coworkers have been very active in synthesizing and developing a portfolio of phosphine ligands for this transformation and other cross-coupling reactions. The ligands used are based on a biaryl skeleton with a phosphorus moiety on the 2 position and another moiety on the other aryl. This class of ligands has proven to be very stable and active for a variety of cross-coupling reactions such as, carbon–carbon, carbon–nitrogen and carbon–oxygen. Stemming from this research, a first generation of phosphine ligand, with dicyclohexyl phosphine dimethylamine was synthesized.1 The phosphine ligand proved to be very versatile and efficient for the cross-coupling of aryl chlorides with alkyl amines using Pd2(dba)3 (Scheme 1). High conversions were observed for a variety of amines and aryls when 3 mol% of the ligand was used with 1.5 mol% of Pd2(dba)3.

Scheme 1
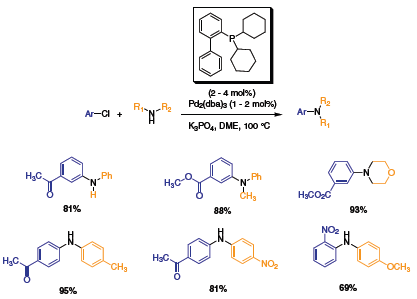
Pursuing the development of ligands for C–N cross couplings, Buchwald and coworkers simplified their ligands by only having the phosphine moiety.2 Using di-tert-butyl and dicyclohexyl phosphines with Pd(OAc)2 and Pd2(dba)3 respectively (Scheme 2 and Scheme 3), Buchwald et al. showed the versatility of these ligands for the C–N cross-coupling of various aryl chlorides with aryl and alkyl amines. Both electron-withdrawing and steric substituents were coupled with good to high yields. In a typical reaction, a ratio of 1 to 2 of Pd(OAc)2 to ligand was used with loading of up to 4 mol%.

Scheme 2
Scheme 3
The next generation of ligands elaborated by Buchwald and coworkers, involved the addition of bulky groups on one of the aryl group. The biaryl molecule was synthesized with dicyclohexylphosphine (XPhos) or di-t-butylphosphine (t-BuXPhos) at the 2 position of one of the phenyl groups and triisopropyl substituted at the 2’, 4’, 6’ position of the second aryl group.3 The addition of bulk to the ligands prevents the formation of the palladacycle and privileges the formation of the mono-ligated palladium complex, the most active species. The substitution also stabilizes the ligands and renders them more active for the C–N cross-coupling reaction. XPhos proved to be very active with Pd2(dba)3 for the coupling of a variety of amines with aryl chloride (Scheme 4). Electron-withdrawing and donating groups on the aryl chloride only affected the yields mildly. The di-t-BuXPhos, the tert-butyl phosphine version of XPhos, showed great activity for the arylation of different pyridines (Scheme 5). Utilizing this ligand with Pd2(dba)3, several dipyridine compounds were synthesized with excellent yields (Table 1). These molecules are used as building blocks for the synthesis of natural products.

Scheme 4

Scheme 5

Table 1
Building on the synthesis of the bulky XPhos and di-t-BuXPhos, Buchwald and co-workers investigated the effect of the bulkiness on the catalytic performance. They synthesized a bulkier ligand with methyl groups at the 2, 3, 4, and 5 position and isopropyl groups at the 2’, 4’, and 6’ position.4 The newly synthesized ligand when used with Pd(OAc)2 (Scheme 6), proved to be very efficient for the cross-coupling of aryl chlorides with aryl alcohols with yields up to 96%. In certain cases it outperformed XPhos and di-t-BuXPhos.

Scheme 6
Stemming from the new generation of Buchwald ligands, a new modified biaryl was synthesized. The diisopropyl substituents were replaced with methoxy groups at the 2’ and 6’ positions.5 The oxygen atoms are believed to stabilize the in-situ formed Pd complex. To test this new ligand, Buchwald et al. ran a series of Suzuki coupling reactions. The activity of the catalyst was highly improved with catalyst loading as low as 0.003 mol% (Scheme 7). To demonstrate the activity of this complex, a variety of bulky aryls were coupled with excellent yields. Following this discovery, the researchers modified the ligand to make it water-soluble. By adding a sodium-sulfonate group at the 5’ position of the bottom ring, the ligand became soluble in aqueous media.6 The new ligand was tested with similar Suzuki cross-coupling reactants in aqueous solution, yielding similar results (Scheme 8). Highly substituted aryls were coupled with high yields.

Scheme 7

Scheme 8
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?