White Catalyst
Introduction
Developed by Professor M. Christina White and co-workers at the University of Illinois–Urbana, the “White catalyst” (684821) has been shown to be an exceptional catalyst for the functionalization of allylic carbon centers.
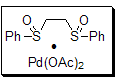
Figure 1.White Catalyst (684821)
The bis-sulfoxide-Pd(II) catalyst participates in numerous reaction manifolds, including inter- and intramolecular allylic C–H oxidation, sequential allylic C–H oxidation/vinylic C–H arylation, and most recently, allylic C–H amination. These useful transformations allow for rapid access to a variety of synthetically useful intermediates such as allylic carboxylates, macrocycles, and amino alcohol derivatives.
Advantages
- Air-stable catalyst
- Access to complex, heteroatom-rich intermediates from simple starting materials
Representative Applications
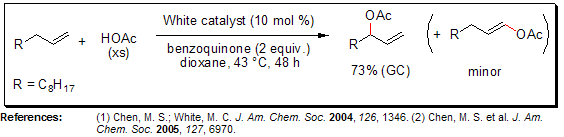
Allylic C–H Oxidation
In 2004, the White group reported sulfoxide-promoted, catalytic Pd(II) / benzoquinonone / HOAc α-olefin allylic oxidation systems, wherein the ratio of linear-to-branched allylic acetates was dependent upon the identity of the sulfoxide present in the reaction (DMSO or bis-sulfoxide ligands). The “White catalyst” (1,2-bis(phenylsulfinyl)ethane]palladium acetate), or phenyl vinyl sulfoxide in the presence of palladium acetate, serve as effective catalysts for allylic C–H oxidation, selectively affording branched allylic acetates.
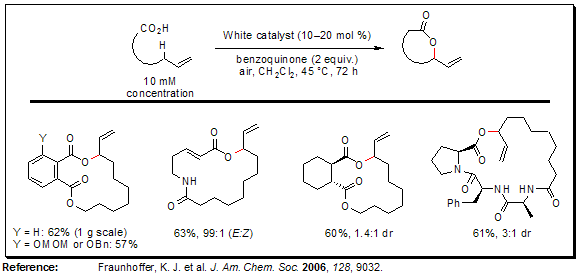
Macrolactonization via Allylic C–H Oxidation
The intermolecular variant of the aforementioned reaction employing linear ω-alkenoic acids is an excellent method for the preparation of macrolactones, and serves as an alternative to hydroxyacid lactonizations so commonly employed. The scope of this novel macrolactonization method is very broad. Aryl, alkyl, and α,β-unsaturated carboxylic acids are all competent nucleophiles, the latter proceeding without olefin isomerization. Finally, the macrolactonizations are exceptionally tolerant of polar functionality (e.g., esters, imides, and amides).
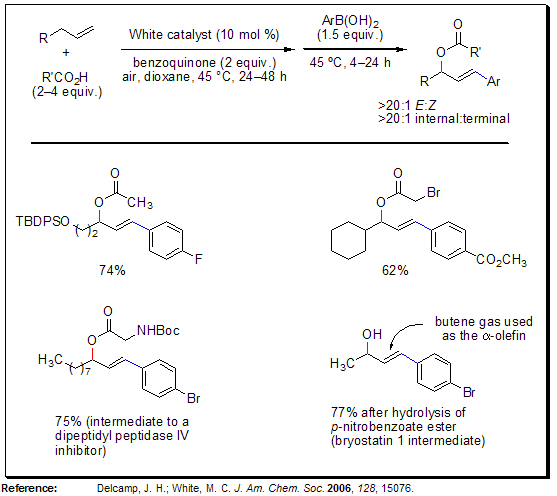
Sequential Allylic C–H Oxidation/Vinylic C–H Arylation
The sequence of allylic C–H oxidation/vinylic C–H arylation of α-olefins furnishes E-arylated allylic esters with high levels of regio- and stereoselectivity. This methodology allows for rapid entry into densely functionalized fragments for complex molecule synthesis from cheap and abundant starting materials (α-olefins, carboxylic acids, arylboronic acids). In contrast to the basic, reductive conditions of the standard Heck reaction, this Pd(II) catalyzed C–H arylation occurs under mildly acidic, oxidative conditions, thus allowing for this use of ortho-substituted and electron-deficient arylboronic acids. Furthermore, a wide range of polar functionality on the α-olefin component is tolerated under the reaction conditions.
Allylic C–H Amination–Preparation of syn-1,2-Amino Alcohol Derivatives
Using the bis-sulfoxide-Pd(II) catalyst, White and co-workers have demonstrated the first general palladium-catalyzed allylic C–H amination reaction, yielding functionalized oxazolidinones from N-tosylcarbamate precursors. The amination reaction is selective for the anti-oxazolidinone diastereomer, thus providing quick entry into medicinally relevant syn-1,2 amino alcohol architectures.
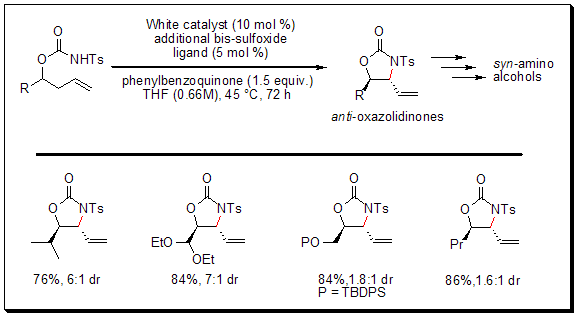
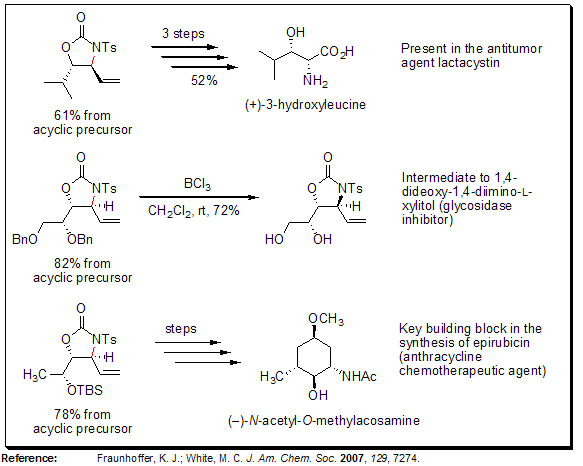
As illustrated below, the anti-oxazolidinones are useful intermediates to several biologically active compounds, including antitumor agents, enzyme inhibitors, and chemotherapeutics. Importantly, this methodology streamlines the syntheses of heteroatom-rich compounds by reducing the overall number of synthetic steps (including functional group manipulations) while at the same time allowing for the late-stage introduction of nitrogen functionality.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?