ECM Gel Matrix: Protocols Using EHS Basement Membrane Extracts
General Handling Guidelines
Neurite Outgrowth Assay Protocol
Cell Invasion Assay Protocol
Angiogenesis Tube Formation Assay Protocol
Pluripotent Stem Cell Culture Protocol
Frequently Asked Questions
The basement membrane is a thin layer of extracellular protein and glycosaminoglycans separating the epithelium from underlying tissue that functions to anchor the epithelium to the supporting connective tissue. Basement membrane extracts (BME) are crude ECM mixtures that may include laminin, collagen IV, entactin, and heparan sulfate proteoglycan, and which are isolated from ECM-rich Engelbreth-Holm-Swarm (EHS) sarcoma tumors in mice. Basement membrane extracts provide a coating substrate for numerous cell types and recapitulates the natural ECM environment found in tissues. ECM Gel is a biological 3D hydrogel isolated from the basement membrane of EHS sarcoma tumors. The product will undergo activated polymerization at 20-40 °C, forming a reconstituted basement membrane. ECM Gel supports numerous in vitro assays such as cell adhesion, neurite outgrowth, cell invasion, cell migration, and angiogenesis tube formation with multiple cell types including epithelial cells, endothelial cells, muscle cells, nerve cells, tumor cells, and stem cells. ECM Gel is cited in over 1000 publications, and is offered in numerous pack sizes in either standard (E1270) or growth factor reduced (E6909) formats. Growth factor-reduced ECM Gel has lower levels of bFGF, EGF, IGF-1, TGF-β, PDGF and NGF cytokines compared to normal ECM Gel.

Figure 1.Structure of the basement membrane. The basement membrane consists of a complex mixture of extracellular matrix proteins (collagen, laminin, fibronectin) and glycosaminoglycans separating the epithelium from underlying connective tissue. ECM Gel Matrix is a basement membrane extract (BME) isolated from EHS tumors rich in ECM proteins.
General Handling Guidelines
- ECM Gel is temperature sensitive. Elevated temperatures will cause the gel to polymerize and solidify rapidly, typically within 5-10 minutes. Thaw frozen ECM Gel overnight at 2-8 °C and always store on ice during use. Dispense gel to wells of a multiwell plate using pipettes, plates, and media pre-cooled to 2-8 °C to avoid polymerization.
- ECM Gel may be diluted up to two fold with cold (2-8 °C) Dulbecco’s Modified Eagle Medium. Dilution may help to reduce viscosity in cases in which the product is still gelatinous in the cold. Gel dilutions should be made before gel is added to the plate. For prolonged manipulations, work should be conducted below 10 °C, on ice.
- Cells may be plated on top of a thin (0.5 mm) gel layer, or cultured inside a 1 mm layer. In the latter application, cells should be added to the gel prior to plating at a recommended density of 3-4 x 104 cells/mL.
- To dissociate cells from the gel, use Dispase (D4818) in PBS without calcium, magnesium, and EDTA, at a concentration of 0.6-2.4 units/mL.
Neurite Outgrowth Assay Protocol
- Neural cells such as PC12 cells (88022401) should be in log phase of growth at 70-80% confluency before starting experiment.
- Pipet 500 μL of cold ECM Gel (E1270, E6909) into the wells. Leave several wells without ECM gel as blank controls.
- Incubate for 5-30 minutes at 37 °C, 5% CO2 for gelation.
- Remove the medium by aspiration.
- Wash the cells twice with 15 mL of sterile PBS (D8527).
- Incubate the cells for 1 minute in 1 mL of Trypsin-EDTA (T4049).
- As the cells round up and start to detach, suspend in 10 mL of medium.
- Determine the cell concentration by hemocytometer using a microscope. Dilute the cell suspension with medium to 2-3 x 105 cells/mL.
- Add 1 mL of PC12 cell suspension to each coated well.
- Incubate at 37 °C, 5% CO2 for 24-48 hours.
- PC12 cells grown on ECM Gel will show extensive neurite outgrowth containing complex 3D neural networks that are observable with an inverted microscope (Figure 2. B, C). PC12 cells that are incubated without ECM Gel will not change morphology and lack neurites (Figure 2. A).

Figure 2.Neurite outgrowth of PC12 rat neuronal cells using ECM Gel Matrix. A) Normal morphology of PC12 cells (88022401) cultured on uncoated plasticware. B, C) PC12 cells grown on ECM Gel Matrix form neurites with complex 3D neural networks after 24-48 hours of culture.
Cell Invasion Assay Protocol
- Thaw ECM Gel (E1270, E6909) overnight at 4 °C and keep on ice.
- Chill Millicell® Insert and plate to 4 °C. Keep on ice.
- Dilute ECM Gel in ice-cold DMEM to a final concentration of 2 mg/mL (See label and Certificate of Analysis for specific ECM Gel concentration).
- Add 100 μL ECM Gel to the upper compartment of the Millicell insert.
- Immediate incubate the plate at 37 °C for 2 hours to allow gel to solidify.
- Wash cells with 1X PBS (D8527) and detach cells using Trypsin-EDTA (T4049).
- Wash cells twice with DMEM and resuspend at 5X105 cells/mL.
- Add DMEM +10% FBS (F2242) to the lower compartment of the plate. Use the volume recommended for the plate. Position the gel-filled insert into the well so that it is submerged in the media.
- Add 1X105 cells to the upper compartment of the insert.
- Incubate the plate at 37 °C for 24hr.
- After the incubation, take out the insert carefully. Remove the cells from the upper compartment with a cotton swab.
- Fix the cells on the lower side of the insert with 5% glutaraldehyde (G5882) for 10 min followed by staining with 1% crystal violet in 2% ethanol (V5265) for 20 minutes.
- Wash with water (W3500) for 3-4 seconds to remove excess dye.
- Dry the insert completely.
- Count number of cells on the lower side of the insert or extract the dye and read on a spectrophotometer to quantify cell invasion.
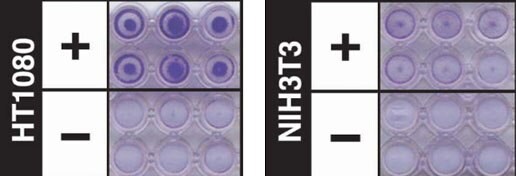
Figure 3.Cell invasion assay using ECM Gel Matrix. Analysis of HT-1080 (85111505) cell invasion through an ECM Gel coated Millicell® insert using a 96-well Cell Invasion Assay (ECM550). Invading cells were visualized by crystal violet staining (purple). NIH3T3 cells (93061524) were used as a non-invasive control.
Angiogenesis Tube Formation Assay Protocol
- HUVEC cells should be in log phase of growth at 70-80% confluency before experiment.
- Serum-starve HUVECS for 3-6 hours prior to tube formation assay.
- Thaw the ECM Gel (E1270, E6909) overnight at 4 °C and keep on ice during protocol.
- Add 100 μL of thawed ECM Gel to each well of a 96-well plate.
- Immediate incubate the plate at 37 °C for 2 hours to allow gel to solidify.
- Detach starved HUVECs using Trypsin-EDTA (T4049) and neutralize with FBS (F2242). Pellet the cells and resuspend in HUVEC media.
- Resuspend the HUVEC cells at 4x105 cells/mL in HUVEC media.
- Dispense 100 µL of the cell suspension to each well of a 96-well plate (4x104 cells/well).
- Incubate the plate at 37 °C, 5% CO2 for 4-6 h.
- Visualize the cells using a light microscope. To quantify angiogenesis, count number of capillary tubes formed, assess the complexity of vascular network and measure tube formation lengths.

Figure 4.Angiogenesis tube formation using ECM Gel Matrix. HUVEC cells stained green using Calcein-AM (17783) after induction of angiogenesis.
Pluripotent Stem Cell Culture Protocol
Expansion of human pluripotent stem cells (ES and iPS Cells) requires cultureware that is coated with prequalified ECM proteins. Below are general guidelines for the coating of 6-well plates using the Stem Cell Qualified ECM Gel Matrix (CC131). All procedures should be performed under aseptic conditions in a biological safety cabinet.
- Thaw the ECM Gel Matrix (CC131) in the 2-8 °C fridge one day before use. Once thawed, maintain ECM Gel on ice at all times and use pre-cooled medium and pipettes to avoid gelling of the product.
- Dilute the ECM Gel Matrix (CC131) 1:80 with cold DMEM/F12 (D6421) medium. Scale up or down according to the volumes required. Below is an example for coating a 6-well plate with 1.5 mL of 1:80 diluted ECM Gel.
To make a 1:20 dilution, add 0.5 mL ECM Gel to 9.5 mL cold DMEM/F12 or DMEM medium into a 15 mL conical tube. Total volume = 10 mL.
A further 4-fold dilution is required to make the final 1:80 dilution. Add 2.5 mL of 1:20 diluted ECM Gel to 7.5 mL cold DMEM/F12 medium. Total volume = 10 mL
NOTE: The recommended dilution is 1:80, however more concentrated Stem Cell Qualified ECM Gel may be used if desired. - Add 1.5 mL of the 1:80 diluted ECM Gel Matrix (CC131) to each well of a 6-well plate. Swirl the culture plates to spread the ECM Gel Matrix evenly across the surface of the plate. Store in a 2 – 8 °C fridge overnight or at least 2 hours in the fridge before use. If not used immediately, parafilm wrap the ECM coated culture plates and store at 2-8 °C until ready to use. Use the ECM coated culture plates within 3-4 days.
- Prior to seeding the cells, bring the plate back to room temperature for 10-15 minutes, remove the coating solution and add 3 mL/well of human ES/iPSC growth media (SCM130). Cells can now be plated onto the newly coated plates.
IMPORTANT: Do not allow the plates to dry out.

Figure 5.Growth of PBMC-derived human iPS cells grown in serum-free media on Stem Cell Qualified ECM Gel Matrix (A) or Matrigel® (B) after 5 passages. Pluripotent morphology of iPSCs grown on ECM Gel is identical to Matrigel® substrates. Cell growth of PBMC-derived human iPSCs grown on Stem Cell Qualified ECM Gel Matrix is comparable to Matrigel® as analyzed by cell counting over 7 pasages (C).
Frequently Asked Questions
Is the ECM Gel derived from cell lysate or actual cell matrix?
The ECM gel is produced from whole EHS tumors (not separated cells). It is the product of a total extraction of the tumors.
Is this product the same as Matrigel® (Corning), Geltrex® (Thermo) or Cultrex® (Trevigen)?
ECM gel is the biological equivalent to these products, manufactured by MilliporeSigma.
Are attachment factors added to ECM Gel?
Product E1270 is extracted from Engelbreth-Holm-Swarm mouse sarcomas with no exogenous additives.
What are the components of the ECM Gel?
The principal components of basement membranes are laminin, collagen IV, entactin, and heparan sulfate proteoglycan (HSPG). The first three are usually present in approximately equimolar amounts, while HSPG is less abundant, but as ECM Gel is a biological extract, precise concentrations of components cannot we cannot state fixed values of relative component concentrations.
What is the calcium concentration in ECM gel?
The approximate concentration of CaCl2 in the ECM solution is 0.265 mg/mL.
Does ECM gel contain antibiotics ?
Our ECM gel is supplemented with gentamicin.
Is it possible to purchase E1270 in DMEM without sodium pyruvate?
The DMEM used in the preparation of E1270 does not contain sodium pyruvate.
Is the ECM gel tested for the presence of mouse retroviruses?
We do not test product E1270 for virus presence. However, several product lots were tested in the past and found free of LDEV, and our raw material supplier Harlan added the LDEV test to their SPF tests list thus the source (mice) are cleaned for this virus. Moreover, by risk assessment, RNA viruses should not survive the manufacturing procedure. Thus, we assume the product is LDEV free.
Will ECM gel perform well for 3D cell culture after freeze-thaw?
We would not recommend thawing and then refreezing the product ECM gel . Alternatively, gels can be cast in plates and stored them at 2-8° C. Seal stored gels securely to prevent dessication and cracking.
The data sheet for E1270 says that it may be diluted up to twofold. What does that mean?
ECM gel may be diluted twofold with cold Dulbecco’s Modified Eagle Medium (DMEM). This means ECM gel may be diluted with an equal volume of cold DMEM.
Does the gel degrade in the presence of cells?
We know that product does not degrade after 7 days of co-incubation with cells.
How can I retrieve my cells from the ECM gel?
Dispase (D4818) is a Bacillus-derived neutral protease that is recommended for recovering cells cultured in ECM gel (Cat. No. E1270). Dispase will yield a single cell suspension far more effectively than trypsin, collagenase, or other proteolytic enzymes. Dispase is gentle and will not damage cells harvested for sub-cultivation or other study.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?