Enzymatic Deglycosylation
Overview
Removal of carbohydrates from glycoproteins is useful for a number of reasons:
- To simplify analysis of the peptide portion of the glycoprotein
- To simplify the analysis of the glycan component
- To remove heterogeneity in glycoproteins for X-ray crystallographic analysis
- To remove carbohydrate epitopes from antigens
- To enhance or reduce blood clearance rates of glycoprotein therapeutics
- To investigate the role of carbohydrates in enzyme activity and solubility
- To investigate ligand binding
- For quality control of glycoprotein pharmaceuticals
Sequential hydrolysis of individual monosaccharides from glycans can be useful for the elucidation of the structure and function of the glycan component. Due to the restraints of the specificity of glycolytic enzymes currently available, sequential hydrolysis of individual monosaccharides is also required in many instances in order to completely remove a glycan component enzymatically. This is particularly true in the enzymatic deglycosylation of many O-linked glycans.
N-Linked Glycan Strategies
Use of the enzyme PNGase F is the most effective method of removing virtually all N-linked oligosaccharides from glycoproteins. The oligosaccharide is left intact and, therefore, suitable for further analysis (the asparagine residue from which the sugar was removed is deaminated to aspartic acid, the only modification to the protein). A tripeptide with the oligosaccharide-linked asparagine as the central residue is the minimal substrate for PNGase F.
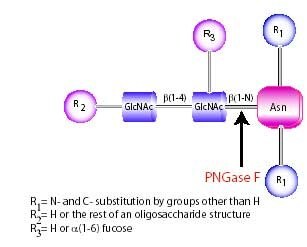
Figure 1.PNGase F
However, oligosaccharides containing a fucose a(1-3)-linked to the asparagine-linked N-acetylglucosamine, commonly found in glycoproteins from plants or parasitic worms, are resistant to PNGase F. Peptide-N-Glycosidase A (PNGase A), isolated from almond meal, must be used in this situation. This enzyme, however, is ineffective when sialic acid is present on the N-linked oligosaccharide. Other commonly used endoglycosidases such as Endoglycosidase H and the Endoglycosidase F series are not suitable for general deglycosylation of N-linked sugars because of their limited specificities and because they leave one N-acetylglucosamine residue attached to the asparagine.
Steric hindrance may slow or inhibit the action of PNGase F at certain glycosylation sites. Denaturation and reduction of the glycoprotein by heating with SDS and 2-mercaptoethanol greatly increases the rate of deglycosylation.
Through sequential deglycosylation of monosaccharides, all complex oligosaccharides can be reduced to the trimannosyldiacetylchitobiose core by selective hydrolysis with a neuraminidase, b-galactosidase, and N-acetylglucosaminidase, available as part of the E-DEGLY kit. Fucosidases may be required in some situations.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?