94950
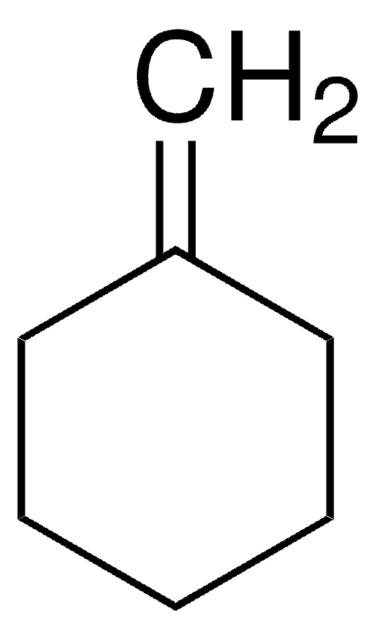
4-Vinyl-1-cyclohexene
analytical standard
Synonyme(s) :
4-Ethenyl-1-cyclohexene, NSC 15760
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Densité de vapeur
3.76 (vs air)
Pression de vapeur
10.2 mmHg ( 25 °C)
Pureté
≥99.5% (GC)
Température d'inflammation spontanée
517 °F
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Indice de réfraction
n20/D 1.463 (lit.)
n20/D 1.464
Point d'ébullition
126-127 °C (lit.)
Pf
−101 °C (lit.)
Densité
0.831 g/mL at 20 °C
0.831 g/mL at 20 °C
0.832 g/mL at 25 °C (lit.)
Application(s)
environmental
petroleum
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
C=CC1CCC=CC1
InChI
1S/C8H12/c1-2-8-6-4-3-5-7-8/h2-4,8H,1,5-7H2
Clé InChI
BBDKZWKEPDTENS-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Produits recommandés
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 3 - Asp. Tox. 1 - Carc. 2 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
60.8 °F - closed cup
Point d'éclair (°C)
16 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique